
As filed with the Securities and Exchange Commission on August 4, 2023.
Registration Statement No. 333-267328
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Post-Effective Amendment No. 2 to
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
XORTX THERAPEUTICS INC.
(Exact name of registrant as specified in its charter)
| British Columbia (State or other jurisdiction of incorporation or organization) | 2834 (Primary Standard Industrial Classification Code Number) | N/A (I.R.S. Employer Identification No.) |
3710 – 33rd Street
NW
Calgary, Alberta, Canada T2L 2M1
(403) 455-7727
(Address, including zip code and telephone number, including area code, of registrant’s principal executive offices)
C T Corporation
1015 15th Street N.W., Suite 1000
Washington, D.C., 20005
(202) 572-3133
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Thomas M. Rose Troutman Pepper Hamilton Sanders LLP 401 9th Street, N.W., Suite 1000 Washington, DC 20004 (202) 274-2950 | Rick Pawluk Fasken Martineau DuMoulin LLP 350 7th Avenue SW, Suite 3400 Calgary AB T2P 3N9 Canada (587) 233-4063 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act.
Emerging growth company x
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.† ¨
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
| † | The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012. |
This Post-Effective Amendment No. 2 relates to the Registration Statement on Form F-1 (File No. 333-267328) (the “Registration Statement”) of XORTX Therapeutics Inc. (the “Company”) originally declared effective by the U.S. Securities and Exchange Commission (“SEC”) on September 22, 2022 (the “Initial Registration Statement”).
The Company is filing this Post-Effective Amendment No. 2 to the Initial Registration Statement (i) to incorporate by reference into the Registration Statement the Company’s Annual Report on Form 20-F for the year ended December 31, 2022, filed with the SEC on April 28, 2023, (ii) to incorporate by reference into the Registration Statement the Unaudited Condensed Interim Consolidated Financial Statements for the three months ended March 31, 2023 and 2022 and the Management Discussion and Analysis for the three months ended March 31, 2023, filed as Exhibits 99.1 and 99.2, respectively, to our Report on Form 6-K furnished to the SEC on May 16, 2023, (iii) to incorporate by reference the Company’s Forms 6-K filed on May 24, 2023, May 30, 2023 and June 30, 2023, and (iv) to include certain other information in the Registration Statement. This Post-Effective Amendment No. 2 contains an updated prospectus relating to the offer and sale of the Company’s common shares issuable upon the exercise of warrants as described herein.
No additional securities are being registered pursuant to this Post-Effective Amendment No. 2.
All filing fees payable in connection with this Registration Statement were paid by the Company at the time of filing of the Initial Registration Statement.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED AUGUST 4, 2023
PRELIMINARY PROSPECTUS

5,000,000 Common Shares Issuable upon
Exercise of Warrants
XORTX Therapeutics Inc.
We are offering 5,000,000 common shares issuable upon exercise of 5,000,000 common share purchase warrants (the “Warrants”) pursuant to this prospectus. The Warrants were offered and sold by us pursuant to a prospectus dated October 4, 2022 as part of a public offering of common share units and pre-funded warrant units. The common share units consisted of 1,400,000 common shares and Warrants to purchase up to 1,400,000 common shares. The pre-funded warrant units consisted of 3,600,000 pre-funded warrants and Warrants to purchase up to 3,600,000 common shares. The common shares and Warrants were immediately separable upon issuance. Each common share unit was sold at a price of $1.00 per common share unit. Each pre-funded warrant unit was sold at a price of $0.9999 and had an exercise price of $0.0001. The Warrants sold in the offering have an exercise price of $1.22 and expire five years from the original date of issuance (October 7, 2027). No securities are being offered pursuant to this prospectus other than the common shares that will be issued upon the exercise of the Warrants.
In order to obtain the common shares offered hereby, holders of Warrants must pay the applicable exercise price per Warrant. We will receive proceeds from the exercises of the Warrants for cash, if any, but not from the sale of the underlying common shares.
There is no established public trading market for the Warrants, and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the Warrants on any national securities exchange. Without an active trading market, the liquidity of the Warrants will be limited.
Our common shares are currently traded under the symbol “XRTX” on the TSX Venture Exchange (the “TSXV”) and on the Nasdaq Capital Market (“Nasdaq”).
We are an “emerging growth company” as defined by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings. However, we have elected not to take advantage of the extended transition period allowed for emerging growth companies for complying with new or revised accounting guidance as allowed by Section 107 of the JOBS Act and Section 7(a)(2)(B) of the Securities Act of 1933, as amended, (the “Securities Act”).
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 10.
We are a “foreign private issuer” as defined under the federal securities laws and, as such, are subject to reduced public company reporting requirements. See “Prospectus Summary – Implications of Being a Foreign Private Issuer.”
Neither the Securities and Exchange Commission, Canadian securities commission nor any domestic or international securities body has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Prospectus dated , 2023
This prospectus is part of a registration statement on Form F-1 that we filed with the Securities and Exchange Commission (“SEC”).
You should read this prospectus and the related registration statement carefully. This prospectus and registration statement contain important information you should consider when making your investment decision. See “Where You Can Find More Information” in this prospectus.
We have not authorized anyone to provide you with information other than that contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give to you. The information contained in this prospectus or any free writing prospectus is accurate only as of the date of this prospectus or such free writing prospectus, regardless of the time of delivery of this prospectus or any free writing prospectus.
We are offering to sell, and seeking offers to buy, securities only in jurisdictions where offers and sales are permitted. We have not taken any action to permit a public offering of our securities or the possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourself about and to observe any restrictions relating to this offering and the distribution of this prospectus.
We express all amounts in this prospectus in United States dollars, except where otherwise indicated. References to “$” are to United States dollars and references to “CAD$” are to Canadian dollars.
Except as otherwise indicated, references in this prospectus to “XORTX,” the “Company,” “we,” “us” and “our” refer to XORTX Therapeutics Inc. and its consolidated subsidiaries.
1
This summary highlights certain information contained elsewhere in this prospectus. This summary does not contain all of the information that may be important to you. You should read and carefully consider the following summary together with the entire prospectus, including all documents incorporated by reference herein. In particular, attention should be directed to the “Risk Factors,” beginning on page 10 of this prospectus and to the sections “Risk Factors,” “Information on the Company,” “Operating and Financial Review and Prospects” and the financial statements and related notes thereto incorporated by reference hereto, in our Annual Report on Form 20-F for the fiscal year ended December 31, 2022, before making an investment decision. See also “Cautionary Note Regarding Forward-Looking Statements,” “Incorporation by Certain Information by Reference” and “Where You Can Find Additional Information” in this prospectus.
Overview
XORTX Therapeutics is a late stage clinical pharmaceutical company focused on identifying, developing and potentially commercializing therapies to treat progressive kidney disease modulated by aberrant purine and uric acid metabolism in orphan (rate) disease indications such as autosomal dominant polycystic kidney disease (“ADPKD”) and type 2 diabetic nephropathy (“T2DN”), as well as acute kidney injury (“AKI”) associated with respiratory virus infection.
Our focus is on developing three therapeutic product candidates to:
| · | slow or reverse the progression of chronic kidney disease in patients at risk of end stage kidney failure; |
| · | address the immediate need of individuals facing AKI associated with respiratory virus infection; and |
| · | Treat patients with type 2 diabetic nephropathy. |
We are also looking to identify other opportunities where our existing and new intellectual property can be leveraged to address health issues.
We believe that our technology is underpinned by well-established research and insights into the underlying biology of aberrant purine metabolism, its health consequences and of oxypurinol, a uric acid lowering agent that works by effectively inhibiting xanthine oxidase. We are developing innovative product candidates that include new or existing drugs that can be adapted to address different disease indications where aberrant purine metabolism and/or elevated uric acid is a common denominator, including polycystic kidney disease, pre-diabetes, insulin resistance, metabolic syndrome, diabetes, diabetic nephropathy, and infection. Oxypurinol, and our proprietary pipeline-in-a-product strategy supported by our intellectual property, established exclusive manufacturing agreements, and proposed clinical trials with experienced clinicians, are focused on building a pipeline of assets to address the unmet medical needs for patients with a variety of serious or life-threatening diseases:
| · | XRx-008, a program for the treatment of ADPKD; |
| · | XRx-101, a program to treat AKI associated with severe respiratory infection and associated health consequences; and |
| · | XRx-225, a program for the treatment of T2DN. |
At XORTX, we aim to redefine the treatment of kidney diseases by developing medications to improve the quality-of-life of patients with life threatening diseases by modulating aberrant purine and uric acid metabolism, including lowering elevated uric acid as a therapy.
Our Proprietary Therapeutic Programs
Our expertise and understanding of the pathological effects of aberrant purine metabolism combined with our understanding of uric acid lowering agent structure and function, has enabled the development of our proprietary therapeutic platforms. These are a complementary suite of therapeutic formulations designed to provide unique solutions for acute and chronic disease. Our therapeutic platforms can be used alone, or in combination, with synergistic activity to develop a multifunctional tailored approach to a variety of disease entities that can address disease in multiple body systems through management of chronic or acute hyperuricemia, immune modulation, and metabolic disease. We continue to leverage these therapeutic platforms to expand our pipeline of novel and next generation drug-based product candidates that we believe could represent significant improvements to the standard of care in multiple acute and chronic cardiovascular diseases and specifically kidney disease.
2
We believe our in-house drug design and formulation capabilities confer a competitive advantage to our therapeutic platforms and are ultimately reflected in our programs. Some of these key advantages are:
Highly modular and customizable
Our platforms can be combined in multiple ways and this synergy can be applied to address acute, intermittent or chronic disease progression. For example, our XRx-101 program for AKI is designed to produce rapid suppression of hyperuricemia then maintain purine metabolism at a low level during viral infection and target management of acute organ injury. Our XRx-008 program is designed for longer term stable chronic oral dosing of xanthine oxidase inhibitors. We believe the capabilities of our formulation technology allow us to manage the unique challenges of cardiovascular and renal disease by modulating, purine metabolism, inflammatory and oxidative state.
Fit-for-purpose
Our platforms can also be utilized to engineer new chemical entities and formulations of those agents that have enhanced properties. For example, our XRx-225 product candidate program, some of the intellectual property for which we license from third parties, represents a potential new class of xanthine oxidase inhibitor with a targeted design to enhance anti-inflammatory activity. The capability of tailoring the potential therapeutic benefit of this class of new agents permits us to identify targets and disease that we wish to exploit and then through formulation design optimize those small molecules and proprietary formulations to maximize potentially clinically meaningful therapeutic effect.
Readily scalable and transferable
Our in-house small molecule and formulations design expertise is positioned to create a steady succession of product candidates that are scalable, efficient to manufacture (by us or a partner or contract manufacturing organization), and produce high production and high purity active pharmaceutical drug product. We believe this will provide a competitive advantage, new intellectual property and opportunity to provide first-in-class products that target unmet medical needs and clinically meaningful quality of life.
Our team’s expertise in uric acid lowering agents, specifically in the development and use of xanthine oxidase inhibitors, has enabled the development of our therapeutic product candidates to treat the symptoms of, and potentially delay the progression of ADPKD, AKI due to respiratory virus infection, and T2DN. There is no guarantee that the Food and Drug Administration (“FDA”) will approve our proposed uric acid lowering agent product candidates for the treatment of kidney disease or the health consequences of diabetes.
Product Candidate Pipeline
Our lead product candidates are XRx-008, XRx-101, and XRx-225. XRx-008 is in preparations for a Phase 3 registration clinical trial, the last stage of clinical development before application for FDA approval. Our XRx-101 program is advancing toward preparing for a “bridging” pharmacokinetic study for the Company’s Phase 3 clinical trial to potentially slow or reverse acute kidney disease in hospitalized individuals with respiratory virus infection. XRx-225 is at the non-clinical stage and advancing toward the clinical development stage.
Products
The Company’s most advanced development program, XRx-008, sometimes referred to by its trademarked name XORLOTM, is a late clinical stage program focused on demonstrating the potential of our novel product candidate for ADPKD. XRx-008 is the development name given to XORTX’s proprietary oral formulation of oxypurinol, and shows increased oral bioavailability compared to oxypurinol alone. XORTX is also developing a second proprietary combination product composed of a uric acid lowering agent administered intravenously, followed by a xanthine oxidase inhibitor - XRx-101 -, for use in treating patients hospitalized with respiratory virus infection and accompanying hyperuricemia with associated AKI.
XORTX is currently evaluating xanthine oxidase inhibitor candidates for the XRx-225 program to potentially treat T2DN as well as developing new chemical entities to address the large unmet medical need.
Patents
XORTX is the exclusive licensee of two U.S. granted patents with claims to the use of all uric acid lowering agents to treat insulin resistance or diabetic nephropathy, and two U.S. patent applications with similar claims for the treatment of metabolic syndrome, diabetes, and fatty liver disease. Counterparts for some of these patent applications have also been submitted in Europe. In both the US and Europe, XORTX owns composition of matter patent applications for unique proprietary formulations of xanthine oxidase inhibitors – U.S. and European patents have been granted. XORTX has also submitted two patent applications to cover the use of uric acid lowering agents for the treatment of the health consequences of respiratory virus infection.
3
XORTX Therapeutics Pipeline:
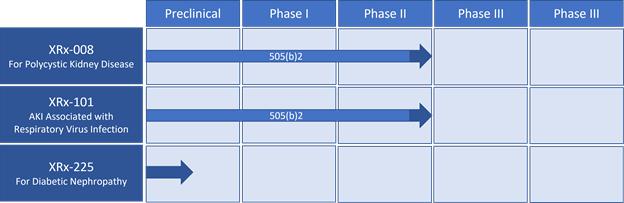
XORTX has held discussions with the FDA, regarding developing oxypurinol using the 505(b)(2) pathway and right of reference to the former oxypurinol New Drug Application (“NDA”). Those discussions indicated that XORTX has the ability to use existing clinical data to bypass conducting a number of its own Phase 1 and Phase 2 studies for XRx-008 and XRx-101 programs. However, we may elect to conduct our own Phase 1 and Phase 2 studies as necessary or required to gain marketing approval in the aforementioned programs.
Our Strategy
Our goal is to apply our interdisciplinary expertise and pipeline-in-a-product strategy to further identify, develop and commercialize novel treatments in renal disease and indications related to health consequences associated with ADPKD. To achieve this objective, we intend to pursue the following strategies:
| 1. | Subject to discussions with FDA, submit an NDA to the FDA following the successful completion of the Phase 3 clinical registration trial of the XRx-008 product candidate program in order to establish a new standard of care for ADPKD. |
| 2. | Maximize the potential of the XRx-008 product candidate program, if approved, through independent commercialization and through opportunistic collaborations with third parties. |
| 3. | Leverage our pipeline-in-a-product strategy, developing additional proprietary formulations leveraging our experience selecting renal indications and complementing our developments through acquisitions or in-licensing opportunities in nephrology and diabetes when opportunities arise. |
For the balance of 2023, XORTX will continue its focus on advancing XORLOTM as part of the XRx-008 program for ADPKD into a Phase 3 registration clinical trial, initiation of special protocol assessment (“SPA”) discussions with the FDA and initiation of pre-commercialization studies to prepare for potential approval of XORLOTM as well as advancing research in other kidney disease applications. To achieve these objectives, XORTX’s action plan includes:
| 1. | Initiate the Phase 3 clinical trial, XRX-OXY-301, to support an application for “Accelerated Approval” of XORLOTM for individuals with ADPKD (the “XRX-OXY-301 Clinical Trial”). The XRX-OXY-301 Clinical Trial is a Phase 3, Multi-Centre, Double-Blind, Placebo Controlled, Randomized Withdrawal Design Study to Evaluate the Efficacy and Safety of a Novel Oxypurinol Formulation in Patients with Progressing Stage 2-4 ADPKD and Coexistent Hyperuricemia. XORTX anticipates that the XRX-OXY-301 Clinical Trial will provide data to support submission of a future NDA application for “Accelerated Approval” to the FDA and Marketing Authorization Application to the European Medicines Agency “EMA.” The XRX-OXY-301 Clinical Trial is planned, subject to additional financing, to start in the second half of 2023 and will enroll individuals with stage 2, 3 or 4 ADPKD accompanied by chronically high uric acid. The objective of the XRX-OXY-301 Clinical Trial is to evaluate the ability of XORLOTM to slow the expansion of total kidney volume over a 12-month treatment period. FDA has granted Orphan Drug Designation status for XRx-008, and confirmation that this program is eligible for Accelerated Approval when Total Kidney Volume (TKV) or estimated glomerular filtration rate (eGFR) clinical data are produced after a 1 year treatment period. |
| 2. | Prepare and Communicate with the FDA and EMA regarding the XRX-OXY-302 Registration trial in ADPKD (the “XRX-OXY-302 Clinical Trial”). The XRX-OXY-302 Clinical Trial is a Phase 3, Multi-Centre, Double-Blind, Placebo Controlled, Randomized Withdrawal Design Study to Evaluate the Efficacy and Safety of a Novel Oxypurinol Formulation in Patients with Progressing Stage 2-4 ADPKD and Coexistent Hyperuricemia with progressing stage 2, 3, or 4 kidney disease. The objective of the XRX-OXY-302 Clinical Trial is to evaluate the safety and effectiveness of XORLOTM for the XRx-008 program over a 24-month treatment period. The aim of the XRX-OXY-302 Clinical Trial is to characterize the ability of xanthine oxidase inhibitors to potentially decrease the rate of decline of glomerular filtration rate. An estimated 300 patients will be enrolled. The XRX-OXY-302 Clinical Trial is planned to start in the second half of 2024, subject to Special Protocol Assessment review by FDA. |
| 3. | Ongoing Chemistry Manufacturing and Control (“CMC”) Work. In parallel with the XRX-OXY-301 and XRX-OXY-302 Clinical Trials, XORTX will be focusing on scale-up, validation and stability testing of clinical drug product supplies of XORLOTM under the Company’s investigational new drug application, as well as future clinical and commercial supplies. All development will be performed according to current Good Manufacturing Practices methodology. This work will be ongoing throughout 2023. |
| 4. | Activities Related to Potential Commercial Launch. In preparation for a possible “Accelerated Approval” NDA filing and approval in 2025 in the US for XORLOTM XRx-008, XORTX will conduct pre-commercialization studies to support in-depth analysis of pricing and/or reimbursement, as well as evaluate product brand name selection, prepare related filings, and conduct other launch preparation activities. This work will be ongoing from 2023 to 2025. |
| 5. | Activities Related to European Registration. XORTX will continue to work with and seek out guidance from the EMA to facilitate the path to potential approval of XORLOTM in the European Union (“EU”), including required clinical studies and reimbursement conditions. This work will be ongoing from 2023 through 2026 and will include a future request for orphan drug status. XORTX intends to seek EMA Orphan Drug Designation status in 2023 to 2024. |
To achieve the above goals, XORTX will continue to pursue non-dilutive and dilutive funding and expand discussions to partner with pharma / biotech companies with a global reach. XORTX will also increase financial and healthcare conference participation to further strengthen and expand its investor base.
Recent Developments
On June 29, 2023, the Company announced the appointment of James Fairbairn as Interim Chief Financial Officer (“CFO”).
FDA has granted Orphan Drug Designation status to XRx-008.
Risk Factors
Our ability to implement our business strategy is subject to numerous risks that you should be aware of before making an investment decision. These risks are described more fully in the sections entitled “Risk Factors” in this prospectus and under “Risk Factors Summary” and “Item 3. Key Information—D. Risk Factors” in our Annual Report on Form 20-F for the year ended December 31, 2022, incorporated by reference herein.
Our Corporate Information
We were incorporated under the laws of Alberta, Canada on August 24, 2012, under the name ReVasCor Inc. and were continued under the Canada Business Corporations Act on February 27, 2013, under the name of XORTX Pharma Corp. Upon completion of a reverse take-over transaction on January 10, 2018, with APAC Resources Inc., a company incorporated under the laws of British Columbia, we changed our name to “XORTX Therapeutics Inc.” and XORTX Pharma Corp. became a wholly-owned subsidiary.
4
Our registered office is located at 3710 – 33rd Street NW, Calgary, Alberta, Canada T2L 2M1 and our telephone number is (403) 455-7727. Our website address is www.xortx.com. The information contained on, or that can be accessed through, our website is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
Implications of Being an Emerging Growth Company
As a company with less than $1.235 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
| · | reduced executive compensation disclosure; |
| · | exemptions from the requirement to hold a non-binding advisory vote on executive compensation, including golden parachute compensation; and |
| · | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002. |
We may take advantage of these provisions until we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earlier to occur of: (1) the last day of our fiscal year following the fifth anniversary of the completion of this offering; (2) the last day of the fiscal year in which we have total annual gross revenue of $1.235 billion or more; (3) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (4) the date on which we are deemed to be a large accelerated filer under the rules of the SEC. We have elected not to take advantage of the extended transition period allowed for emerging growth companies for complying with new or revised accounting guidance as allowed by Section 107 of the JOBS Act and Section 7(a)(2)(B) of the Securities Act.
5
We report under the Securities Exchange Act of 1934, as amended (“Exchange Act”), as a non-U.S. company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we continue to qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| · | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations with respect to a security registered under the Exchange Act; |
| · | the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and |
| · | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial statements and other specified information, and current reports on Form 8-K upon the occurrence of specified significant events, although we report our results of operations on a quarterly basis under the Canadian securities laws. |
Both foreign private issuers and emerging growth companies are also exempt from certain more stringent executive compensation disclosure rules. Thus, even if we no longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt from the more stringent compensation disclosures required of companies that are neither an emerging growth company nor a foreign private issuer.
We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents, and any one of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents, (ii) more than 50% of our assets are located in the United States or (iii) our business is administered principally in the United States.
In this prospectus, we have taken advantage of certain of the reduced reporting requirements as a result of being an emerging growth company and a foreign private issuer. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold equity securities.
6
The Offering
Securities offered |
Up to 5,000,000 common shares, no par value per share (each a “common share”) are issuable upon exercise of the Warrants at an exercise price per common share of $1.22. | |
| Description of Warrants | The Warrants were offered and sold by us pursuant to a prospectus dated October 4, 2022, as part of a public offering of common share units and pre-funded warrant units. The common share units consisted of 1,400,000 common shares and Warrants to purchase up to 1,400,000 common shares. The pre-funded warrant units consisted of 3,600,000 pre-funded warrants and Warrants to purchase up to 3,600,000 common shares. The common shares and Warrants were immediately separable upon issuance. Each common share unit was sold at a price of $1.00 per common share unit. Each pre-funded warrant unit was sold at a price of $0.9999 and had an exercise price of $0.0001. The Warrants sold in the offering have an exercise price of $1.22 and expire five years from the original date of issuance (October 7, 2027). | |
| Limitations on beneficial ownership | Under the Warrants, a holder (together with its affiliates) may not exercise any portion of a Warrant to the extent that the holder would own more than 4.99% of our outstanding common shares outstanding immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding common shares after exercising the holder’s Warrants up to 9.99% of the number of our common shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Warrants. | |
| Common shares to be outstanding after this offering, assuming exercise of all of the Warrants | 22,989,687 shares | |
| Use of proceeds | We may receive proceeds from the exercise of the warrants if exercised for cash, but not from the sale of the underlying common shares. We intend to use the net proceeds of the warrant exercises to fund our ongoing research and development activities, and for working capital and general corporate purposes. See “Use of Proceeds”. | |
| Nasdaq trading symbol | “XRTX” | |
| No Listing of Warrants | We do not intend to apply for listing of the Warrants on any national securities exchange or trading system. | |
| Risk Factors | Please refer to “Risk Factors” in this prospectus and under “Risk Factors Summary” and “Item 3. Key Information—D. Risk factors” in our Annual Report on Form 20-F for the year ended December 31, 2022, incorporated by reference herein, and other information included or incorporated by reference in this prospectus for a discussion of factors you should carefully consider before investing in our common shares. |
7
The number of common shares to be outstanding after this offering is based on 17,989,687 common shares outstanding as of March 31, 2023, and excludes:
| · | 1,039,335 common shares issuable upon the exercise of outstanding options to issue common shares, as of March 31, 2023, at a weighted average exercise price of CAD$2.03 per common share; and |
| · | 5,579,796 common shares issuable upon the exercise of outstanding common share warrants (excluding the Warrants), as of March 31, 2023, at a weighted-average exercise price of $3.59 per common share. |
Unless otherwise indicated, all information in this prospectus reflects or assumes the Warrants have all been exercised.
8
On June 29, 2023, we announced the appointment of James Fairbairn as Interim CFO. On July 31, 2023, Amar Keshri ceased to be employed by the Company.
Pursuant to Mr. Fairbairn’s appointment as our interim CFO, we entered into a consulting agreement with 1282803 Ontario Inc., pursuant to which we appointed Mr. Fairbairn to act as our consultant, including in the capacity as our interim CFO (the “Fairbairn Consulting Agreement”). The terms of the Fairbairn Consulting Agreement are effective from July 3, 2023 on a continuous basis until terminated by either party and provided for Mr. Fairbairn’s services as an independent consultant. Under the Fairbairn Consulting Agreement, Mr. Fairbairn will act as our CFO for a term of one-year, which shall automatically renew, but is cancellable by either party on 90 days’ notice. In return for services as Interim CFO, we will pay a fee of CAD$205,540 annually. In addition, Mr. Fairbairn is to provide certain strategic financial guidance for a one-year term in exchange for the grant of 30,000 stock options, which shall vest equally over 36 months. The Fairbairn Consulting Agreement provides that Mr. Fairbairn is eligible to participate in our stock option plan and is eligible for a discretionary bonus of up to 30% of the annual base consulting fees. Either party may terminate the Fairbairn Consulting Agreement upon not less than 30 days written notice. In the event that we were to terminate the agreement, we would be required to pay a single lump-sum termination payment equal to one month of the base consulting fee as in effect at the time of termination.
9
Any investment in our securities involves a high degree of risk. You should carefully consider the risks described below and in “Risk Factors Summary” and “Item 3. Key Information - D. Risk factors” in our Annual Report on Form 20-F for the year ended December 31, 2022, incorporated by reference herein, and all of the information included or incorporated by reference in this prospectus before deciding whether to purchase our securities. The risks and uncertainties described below are not the only risks and uncertainties we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. If any of the events or circumstances described in the following risk factors actually occur, our business, financial condition and results of operations would suffer. In that event, the price of our common shares could decline, and you may lose all or part of your investment. The risks discussed below also include forward-looking statements and our actual results may differ substantially from those discussed in these forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements.”
Risks Related to this Offering
We will have broad discretion in how we use the proceeds, and we may use the proceeds in ways in which you and other stockholders may disagree.
We intend to use the net proceeds we receive from this offering, if any, to fund our ongoing research and development activities, and for working capital and general corporate purposes. Our management will have broad discretion in the application of the proceeds from this offering and could spend the proceeds in ways that do not necessarily improve our operating results or enhance the value of our common shares.
If you purchase common shares in this offering by exercising warrants, you will suffer immediate dilution of your investment.
The public offering price of our common shares is substantially higher than the as adjusted net tangible book value per common share. Therefore, if you purchase common shares in this offering by exercising warrants, you will pay a price per common share that substantially exceeds our as adjusted net tangible book value per common share after this offering. To the extent outstanding options are exercised, you will incur further dilution. Based on the exercise price per common share of the Warrants, you will experience immediate dilution of $0.57 per common share, representing the difference between our as adjusted net tangible book value per common share after giving effect to this offering and the applicable exercise price. See “Dilution.”
10
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements contained in this Annual Report constitute forward-looking statements. These statements relate to future events or the Company’s (as defined herein) future performance. All statements other than statements of historical fact are forward-looking statements. The use of any of the words “anticipate”, “plan”, “contemplate”, “continue”, “estimate”, “expect”, “intend”, “propose”, “might”, “may”, “will”, “shall”, “project”, “should”, “could”, “would”, “believe”, “predict”, “forecast”, “pursue”, “potential” and “capable” and similar expressions are intended to identify forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results or events to differ materially from those anticipated in such forward-looking statements. No assurance can be given that these expectations will prove to be correct and such forward-looking statements included in this Annual Report should not be unduly relied upon. These statements speak only as of the date of this Annual Report. In addition, this Annual Report may contain forward-looking statements and forward-looking information attributed to third party industry sources.
In particular, forward-looking statements in this Annual Report include, but are not limited to, statements about:
| · | our ability to obtain additional financing; |
| · | the accuracy of our estimates regarding expenses, future revenues and capital requirements; |
| · | the success and timing of our preclinical studies and clinical trials; |
| · | our ability to obtain and maintain regulatory approval of XRx-008, also sometimes referred to by its trademarked name XORLOTM. XORTX’s proprietary formulation of oxypurinol, and any other product candidates we may develop, and the labeling under any approval we may obtain; |
| · | regulatory approvals and discussions and other regulatory developments in the United States, the EU and other countries; |
| · | the performance of third-party manufacturers and contract research organizations; |
| · | our plans to develop and commercialize our product candidates, if approved; |
| · | our plans to advance research in other kidney disease applications; |
| · | our ability to obtain and maintain intellectual property protection for our product candidates; |
| · | the successful development of our sales and marketing capabilities; |
| · | the potential markets for our product candidates and our ability to serve those markets; |
| · | the rate and degree of market acceptance of any future products; |
| · | the success of competing drugs that are or become available; and |
| · | the loss of key scientific or management personnel. |
All forward-looking statements, including, without limitation, our examination of historical operating trends, are based upon our current expectations and various assumptions. Certain assumptions made in preparing the forward-looking statements include:
| · | the availability of capital to fund planned expenditures; |
| · | prevailing regulatory, tax and environmental laws and regulations; |
| · | the ability to secure necessary personnel, equipment, supplies and services; |
| · | our ability to manage our growth effectively; |
| · | the absence of material adverse changes in our industry or the global economy; |
| · | trends in our industry and markets; |
11
| · | our ability to maintain good business relationships with our strategic partners; |
| · | our ability to comply with current and future regulatory standards; |
| · | our ability to protect our intellectual property rights; |
| · | our continued compliance with third-party license terms and the non-infringement of third-party intellectual property rights; |
| · | our ability to manage and integrate acquisitions; and |
| · | our ability to raise sufficient debt or equity financing to support our continued growth. |
We believe there is a reasonable basis for our expectations and beliefs, but they are inherently uncertain. We may not realize our expectations, and our beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements.
12
We express all amounts in this prospectus in United States dollars, except where otherwise indicated. References to “$” are to United States dollars and references to “CAD$” are to Canadian dollars. The following table sets forth, for the periods indicated, average rate of exchange for one U.S. dollar, expressed in Canadian dollars, for the years ended December 31, 2022, 2021 and 2020, as supplied by the Bank of Canada:
| Year Ended | Average | |||
| December 31, 2022 | 1.3013 | |||
| December 31, 2021 | 1.2535 | |||
| December 31, 2020 | 1.3415 | |||
On June 30, 2023, the Bank of Canada average daily rate of exchange was $1.00 = CAD$1.3240.
13
MARKET, INDUSTRY AND OTHER DATA
Unless otherwise indicated, information contained in, and incorporated into, this prospectus concerning our industry and the market in which we operate, including our market position, market opportunity and market size, is based on information from various third-party sources not prepared at the direction of the Company, such as industry publications, and assumptions that we have made based on such data and other similar sources and on our knowledge of the markets for our products. These data involve a number of assumptions and limitations. We believe that this data is accurate and that its estimates and assumptions are reasonable, but there can be no assurance as to the accuracy or completeness of this data. We have not independently verified any of the data from third-party sources referred to in, or incorporated into, this prospectus or analyzed or verified the underlying studies or surveys relied upon or referred to by such sources, or ascertained the underlying economic assumptions relied upon or referred to by such sources.
In addition, projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate is necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section entitled “Risk Factors” and discussed elsewhere in this prospectus, as well as in “Risk Factors Summary” and “Item 3. Key Information - D. Risk factors” in our Annual Report on Form 20-F for the year ended December 31, 2022, incorporated by reference herein. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
14
To the extent that the Warrants are exercised for cash, we will receive the gross cash proceeds from such exercise of up to a total potential of approximately $6,100,000 million, based on the current exercise price of the Warrants. We cannot predict when or if any of the Warrants will be exercised, and it is possible that the warrants may expire and never be exercised.
We intend to use the net proceeds from the issuance of the securities for working capital and general corporate purposes. Such purposes may include research and development expenditures and capital expenditures.
Our management will have broad discretion in the application of the net proceeds of this offering, and investors will be relying on our judgment regarding the application of the net proceeds. In addition, we might decide to postpone or not pursue certain preclinical activities or clinical trials if the net proceeds from this offering and our other sources of cash are less than expected.
Pending their use, we plan to invest the net proceeds of any Warrant exercises in short-and intermediate-term interest-bearing investments.
15
We have never paid any dividends on our common shares or any of our other securities. We currently intend to retain any future earnings to finance the growth and development of our business, and we do not anticipate that we will declare or pay any cash dividends in the foreseeable future. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements, restrictions under any future indebtedness and other factors the Board of Directors deems relevant.
16
CAPITALIZATION AND INDEBTEDNESS
The following table sets forth our cash as well as capitalization as of March 31, 2023:
| · | on an actual basis; |
| · | on an as adjusted basis to give effect to our issuance of 5,000,000 common shares offered hereby upon exercise of the Warrants at an exercise price per common share of $1.22; |
| · | Canadian Dollar amounts have been translated into U.S. Dollars based on the March 31, 2023, daily rate of exchange, which was $1.00 = CAD$1.3533 or CAD$1.00 = $0.7389 as reported by the Bank of Canada and have been provided solely for the convenience of the reader. |
You should read this table together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in “Item 5. Operating and Financial Review and Prospects” and our financial statements and related notes thereto included in “Item 18. Financial Statements” included in our Annual Report on Form 20-F for the year ended December 31, 2022, incorporated by reference herein.
| As of March 31, 2023 | ||||||||
| Pro forma as | ||||||||
| Actual | adjusted | |||||||
| (In thousands, except share data) | ||||||||
| Cash | $ | 7,908 | $ | 14,008 | ||||
| Equity | ||||||||
| Share capital | $ | 17,057 | $ | 23,157 | ||||
| Common shares, unlimited authorized shares, without par value; 17,989,687 shares issued and outstanding, actual; 22,989,687 shares issued and outstanding, pro forma as adjusted | ||||||||
| Share-based payments, warrant reserve and other | $ | 9,559 | $ | 9,559 | ||||
| Obligation to Issue Shares | $ | 25 | $ | 25 | ||||
| Accumulated other comprehensive (loss) income | $ | (53 | ) | $ | (53 | ) | ||
| Deficit | $ | (17,529 | ) | $ | (17,529 | ) | ||
| Total Equity | $ | 9,059 | $ | 15,159 | ||||
| Total Capitalization | $ | 9,059 | $ | 15,159 | ||||
The number of common shares to be outstanding after this offering is based on an aggregate of 17,989,687 shares outstanding as of March 31, 2023. The table above excludes:
| · | 1,039,335 common shares issuable upon the exercise of outstanding options to issue common shares, as of March 31, 2023, at a weighted-average exercise price of CAD$2.03 per share; and | |
| · | 5,579,796 common shares issuable upon the exercise of outstanding common share purchase warrants, as of March 31, 2023, at a weighted-average exercise price of $3.59 per share. |
For additional information regarding our share capital and the terms of the Warrants, see “Description of Share Capital” and “Description of Warrants.”
17
If you exercise Warrants in this offering for our common shares, your interest will be diluted to the extent of the difference between the price per common share you will pay and the as adjusted net tangible book value per common share after the exercise.
As of March 31, 2023, we had a net tangible book value of $8,890 million, corresponding to a net tangible book value of $0.49 per common share. Net tangible book value per share represents the amount of our total assets less our total liabilities, excluding intangible assets, divided by 17,989,687, the total number of our common shares outstanding as of March 31, 2023.
Assuming that we issue 5,000,000 common shares upon exercise of the Warrants at an exercise price per common share of $1.22, our as adjusted net tangible book value estimated as of March 31, 2023 would have been $14,991 million, representing $0.65 per common share. This represents an immediate increase in net tangible book value of $0.16 per common share to existing shareholders and an immediate dilution in net tangible book value of $0.57 per common share to new investors acquiring common shares upon the exercise of the Warrants. Dilution for this purpose represents the difference between the exercise price per common share paid upon exercise of the Warrants and net tangible book value per common share immediately after the exercise.
The following table illustrates this dilution to new investors for holders of Warrants.
| Warrant holder |
||||
| Exercise price per common share | $ | 1.22 | ||
| Net tangible book value per common share as of March 31, 2023 | $ | 0.49 | ||
| Increase in net tangible book value per common share attributable to new investors | $ | 0.16 | ||
| As adjusted net tangible book value per common share after the exercise | $ | 0.65 | ||
| Dilution per common share to new investors | $ | 0.57 | ||
| Percentage of dilution in net tangible book value per common share for new investors | 46.72 | % | ||
The above discussion and table are based on 17,989,687 common shares outstanding as of March 31, 2023, and excludes:
| · | 1,039,335 common shares issuable upon the exercise of outstanding options to issue common shares, as of March 31, 2023, at a weighted-average exercise price of CAD$2.03 per share; and | |
| · | 5,579,796 common shares issuable upon the exercise of outstanding common share purchase warrants, as of March 31, 2023, at a weighted-average exercise price of $3.59 per share. |
To the extent that outstanding options or warrants are exercised, you may experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities may result in further dilution to our shareholders.
Canadian Dollar amounts have been translated into U.S. Dollars based on the March 31, 2023, daily rate of exchange, which was $1.00 = CAD$1.3533 or CAD$1.00 = $0.7389 as reported by the Bank of Canada and have been provided solely for the convenience of the reader.
18
Our common shares are listed on Nasdaq and the TSXV under the symbol “XRTX”.
TRANSFER AGENT, REGISTRAR AND AUDITOR
The transfer agent and registrar for our common shares is TSX Trust Company at its principal office in Toronto, Canada. Our co-transfer agent is Continental Stock Transfer & Trust Company.
Smythe LLP, located at 1700 — 475 Howe Street, Vancouver, British Columbia, Canada V6C 2B3 is our independent registered public accounting firm and has been appointed as our independent auditor.
19
General
The following is a summary of the material rights of our share capital as contained in our notice of articles and articles and any amendments thereto. This summary is not a complete description of the share rights associated with our capital stock. For more detailed information, please see our notice of articles and articles, which are filed as exhibits to the registration statement of which this prospectus forms a part.
Common Shares
Outstanding Shares
Our authorized share capital consists of an unlimited number of common shares, each without par value.
As of March 31, 2023, we had 795,859 common shares issuable pursuant to exercisable outstanding stock options, 243,476 common shares issuable pursuant to outstanding options that are not currently exercisable, 10,579,796 common shares issuable upon the exercise of outstanding common share warrants, and we had approximately 15 holders of record of our common shares.
Voting Rights
Under our articles, the holders of our common shares are entitled to one vote for each common share held on all matters submitted to a vote of the shareholders, including the election of directors. Our notice of articles and articles do not provide for cumulative voting rights. Because of this, the holders of a plurality of the common shares entitled to vote in any election of directors can elect all of the directors standing for election, if they so choose.
Dividends
Subject to priority rights that may be applicable to any then outstanding common shares, and the applicable provisions of the Business Corporation Act British Columbia (“BCBCA”), holders of our common shares are entitled to receive dividends, as and when declared by our Board, in their sole discretion as they see fit. For more information, see the section titled “Dividend Policy.”
Liquidation
In the event of our liquidation, dissolution or winding up, holders of our common shares are entitled to share ratably in the net assets legally available for distribution to shareholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then outstanding preferred shares.
Rights and Preferences
Our common shares contain no pre-emptive or conversion rights and have no provisions for redemption or repurchase for cancellation, surrender or sinking or purchase funds. There are no provisions in our notice of articles and articles requiring holders of common shares to contribute additional capital. The rights, preferences and privileges of the holders of our common shares are subject to and may be adversely affected by the rights of the holders of any series of new preferred shares that may be created, authorized, designated, and issued in the future.
Fully Paid and Non-assessable
All of our outstanding common shares are, and the common shares to be issued pursuant to this Prospectus, when paid for, will be fully paid and non-assessable.
20
The following is a summary of certain terms and provisions of the Warrants. This summary is not complete and is subject to, and qualified in its entirety by, the provisions of the Warrants, the form of which is included as exhibit 4.6 to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of Warrants for a complete description of the terms and conditions of the Warrants.
Duration and Exercise Price
Each Warrant included had an initial exercise price equal to $1.22 per common share.
The Warrants were immediately exercisable and expire on the fifth anniversary of the original issuance date (October 7, 2027). The exercise price and number of common shares issuable upon exercise is subject to appropriate adjustment in the event of share dividends, share splits, reorganizations or similar events affecting our common shares and the exercise price. The Warrants were sold in common share units and pre-funded warrant units, with the common share unit consisting of one common share and one Warrant and each pre-funded warrant unit consisting of one pre-funded warrant and one Warrant.
Cashless Exercise
If, at the time a holder exercises its Warrants, a registration statement registering the issuance of the shares of common shares underlying the under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of common shares determined according to a formula set forth in the Warrants.
Exercisability
The Warrants are exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of common shares purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the Warrant to the extent that the holder would own more than 4.99% of the outstanding common shares immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding common shares after exercising the holder’s Warrants up to 9.99% of the number of common shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Warrants. Purchasers of Warrants in the 2021 public offering could also elect, prior to the issuance of the Warrants, to have the initial exercise limitation set at 9.99% of our outstanding common shares.
Fractional Shares
No fractional common shares will be issued upon the exercise of the Warrants. Rather, the number of common shares to be issued will be rounded up to the nearest whole number, or the Company shall pay a cash adjustment in respect of the fractional share.
Transferability
Subject to applicable laws, the Warrants may be offered for sale, sold, transferred or assigned without our consent. There is currently no trading market for the Warrants.
Exchange Listing
There is no trading market available for the Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the Warrants on any securities exchange or nationally recognized trading system.
Right as a Shareholder
Except as otherwise provided in the Warrants or by virtue of such holder’s ownership of common shares, the holders of the Warrants do not have the rights or privileges of holders of our common shares, including any voting rights, until they exercise their Warrants.
Fundamental Transaction
In the event of a fundamental transaction, as described in the Warrants and generally including any reorganization, recapitalization or reclassification of our common shares, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common shares, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common shares, the holders of the Warrants will be entitled to receive upon exercise of the Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Warrants immediately prior to such fundamental transaction.
21
Material Canadian Federal Income Tax Considerations
The following is, as of the date of this prospectus, a general summary of the principal Canadian federal income tax considerations under the Income Tax Act (Canada), or the Canadian Tax Act, generally applicable to an investor who acquires common share units pursuant to this offering and who, for the purposes of the Canadian Tax Act and at all relevant times, deals at arm’s length with the Company and the underwriters, is not affiliated with the Company or the underwriters and who acquires and holds the common shares, or Warrants as capital property, or a Holder. Generally, the common shares and Warrants will be considered to be capital property to a Holder thereof provided that the Holder does not use the common shares in the course of carrying on a business of trading or dealing in securities and such Holder has not acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder (i) that is a “financial institution” for the purposes of the mark-to-market rules contained in the Canadian Tax Act; (ii) that is a “specified financial institution” as defined in the Canadian Tax Act; (iii) if an interest in such a Holder is a “tax shelter” or a “tax shelter investment,” each as defined in the Canadian Tax Act; (iv) a holder that reports its “Canadian tax results,” as defined in the Canadian Tax Act, in a currency other than Canadian currency; or (v) that has or will enter into a “derivative forward agreement” or a “synthetic disposition arrangement”, as those terms are defined in the Canadian Tax Act, with respect to the common shares and Warrants. Such Holders should consult their own tax advisors with respect to the consequences of acquiring common share units.
Additional considerations, not discussed herein, may be applicable to a Holder that (i) is a corporation resident in Canada and (ii) is (or does not deal at arm’s length for the purposes of the Canadian Tax Act with a corporation resident in Canada that is), or becomes as part of a transaction or event or series of transactions or events that includes the acquisition of the common share units, controlled by a corporation that is not resident in Canada for purposes of the “foreign affiliate dumping” rules in section 212.3 of the Canadian Tax Act. Such Holders should consult their own tax advisors with respect to the consequences of acquiring common share units.
This summary is based upon the current provisions of the Canadian Tax Act and the regulations thereunder, or the Regulations, in force as of the date hereof and the Company’s understanding of the current published administrative and assessing practices of the Canada Revenue Agency, or the CRA. This summary takes into account all specific proposals to amend the Canadian Tax Act and the Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof, or the Tax Proposals, and assumes that the Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all. This summary does not otherwise take into account any changes in law or in the administrative policies or assessing practices of the CRA, whether by legislative, governmental or judicial decision or action, nor does it take into account or consider any provincial, territorial or foreign income tax considerations, which considerations may differ significantly from the Canadian federal income tax considerations discussed in this summary.
This summary is of a general nature only, is not exhaustive of all possible Canadian federal income tax considerations and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Holder. This summary does not address the deductibility of interest expense incurred or paid by a Holder that has borrowed money in connection with the acquisition of common share units pursuant to this offering. Holders should consult their own tax advisors with respect to their particular circumstances.
All amounts in a currency other than the Canadian dollar relevant in computing a Holder’s liability under the Canadian Tax Act with respect to the acquisition, holding or disposition of common shares and Warrants must generally be converted into Canadian dollars using the single daily exchange rate quoted by the Bank of Canada for the day on which the amount arose or such other rate of exchange that is acceptable to the CRA.
22
Residents of Canada
The following section of this summary applies to a Holder who, for the purposes of the Canadian Tax Act, is or is deemed to be resident in Canada at all relevant times, or a Canadian Resident Holder. Certain Canadian Resident Holders whose common shares might not constitute capital property may in certain circumstances make an irrevocable election in accordance with subsection 39(4) of the Canadian Tax Act to deem the common shares, and every other “Canadian security” as defined in the Canadian Tax Act, held by such Canadian Resident Holder, in the taxation year of the election and each subsequent taxation year to be capital property. Canadian Resident Holders should consult their own tax advisors regarding this election.
Dividends
Dividends received or deemed to be received on the common shares will be included in computing a Canadian Resident Holder’s income. In the case of an individual (other than certain trusts), such dividends will be subject to the gross-up and dividend tax credit rules normally applicable in respect of “taxable dividends” received from “taxable Canadian corporations” (each as defined in the Canadian Tax Act). An enhanced dividend tax credit will be available to individuals in respect of “eligible dividends” designated by the Company to the Canadian Resident Holder in accordance with the provisions of the Canadian Tax Act.
Dividends received or deemed to be received by a corporation that is a Canadian Resident Holder on the common shares must be included in computing its income but generally will be deductible in computing its taxable income. In certain circumstances, subsection 55(2) of the Canadian Tax Act will treat a taxable dividend received by a Canadian Resident Holder that is a corporation as proceeds of disposition or a capital gain. A Canadian Resident Holder that is a corporation should consult its own tax advisors having regard to its own circumstances. A Canadian Resident Holder that is a “private corporation” as defined in the Canadian Tax Act and certain other corporations controlled, by or for the benefit of an individual (other than a trust) or a related group of individuals (other than trusts) generally will be liable to pay a 38 1/3% refundable tax under Part IV of the Canadian Tax Act on dividends received or deemed to be received on the common shares to the extent such dividends are deductible in computing taxable income. Such refundable tax will generally be refunded to a corporate Canadian Resident Holder at the rate of 38 1/3% of taxable dividends paid while it is a private corporation.
Expiry of Warrants
In the event of the expiry of an unexercised Warrant, a Canadian Resident Holder will be considered to have disposed of such Warrant for nil proceeds and will accordingly realize a capital loss equal to the Canadian Resident Holder’s adjusted cost base of such Warrant immediately before that time. For a description of the tax treatment of capital losses, see “Capital Gains and Losses”, below.
Exercise of Warrants
No gain or loss will be realized by a Canadian Resident Holder on the exercise of a Warrant to acquire common shares. When a Warrant is exercised, the Canadian Resident Holder’s cost of the common shares acquired thereby will be equal to the adjusted cost base of the Warrant to the Canadian Resident Holder, immediately before that time, plus the amount paid on the exercise of the Warrant. For the purpose of computing the adjusted cost base of each common share acquired on the exercise of a Warrant, the cost of such common share must be averaged with the adjusted cost base to the Canadian Resident Holder of all other common shares held as capital property immediately before the exercise of the Warrant.
Dividends received or deemed to be received by a corporation that is a Canadian Resident Holder on the common shares must be included in computing its income but generally will be deductible in computing its taxable income. In certain circumstances, subsection 55(2) of the Canadian Tax Act will treat a taxable dividend received by a Canadian Resident Holder that is a corporation as proceeds of disposition or a capital gain. A Canadian Resident Holder that is a corporation should consult its own tax advisors having regard to its own circumstances. A Canadian Resident Holder that is a “private corporation” as defined in the Canadian Tax Act and certain other corporations controlled, by or for the benefit of an individual (other than a trust) or a related group of individuals (other than trusts) generally will be liable to pay a 38 1/3% refundable tax under Part IV of the Canadian Tax Act on dividends received or deemed to be received on the common shares to the extent such dividends are deductible in computing taxable income. Such refundable tax will generally be refunded to a corporate Canadian Resident Holder at the rate of 38 1/3% of taxable dividends paid while it is a private corporation.
Dispositions of Common Shares or Warrants
Upon a disposition (or a deemed disposition) of a common share, a Canadian Resident Holder generally will realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition of such common share, net of any reasonable costs of disposition, are greater (or are less) than the adjusted cost base of such common share to the Canadian Resident Holder. The tax treatment of capital gains and capital losses is discussed in greater detail below under the subheading “Capital Gains and Capital Losses.”
23
The adjusted cost base to a Canadian Resident Holder of a common share acquired pursuant to this offering will be averaged with the adjusted cost base of any other of the Company’s common shares held by such Canadian Resident Holder as capital property for the purposes of determining the Canadian Resident Holder’s adjusted cost base of each common share.
Capital Gains and Capital Losses
Generally, a Canadian Resident Holder is required to include in computing its income for a taxation year one-half of the amount of any capital gain (a “taxable capital gain”) realized in the year. Subject to and in accordance with the provisions of the Canadian Tax Act, a Canadian Resident Holder is required to deduct one-half of the amount of any capital loss (an “allowable capital loss”) realized in a taxation year from taxable capital gains realized in the year by such Canadian Resident Holder. Allowable capital losses in excess of taxable capital gains may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any following taxation year against taxable capital gains realized in such year to the extent and under the circumstances described in the Canadian Tax Act.
The amount of any capital loss realized on the disposition or deemed disposition of common shares by a Canadian Resident Holder that is a corporation may be reduced by the amount of dividends received or deemed to have been received by it on such shares or shares substituted for such shares to the extent and in the circumstances specified by the Canadian Tax Act. Similar rules may apply where a Canadian Resident Holder that is a corporation is a member of a partnership or beneficiary of a trust that owns such shares or that itself is a member of a partnership of a beneficiary of a trust that owns such shares. Canadian Resident Holders to whom these rules may be relevant should consult their own tax advisors.
A Canadian Resident Holder that is throughout the relevant taxation year a “Canadian-controlled private corporation” as defined in the Canadian Tax Act may also be liable to pay an additional refundable tax on its “aggregate investment income” for the year which will include taxable capital gains. The rate of the refundable tax is 10 2/3% for taxation years beginning after 2015. Such refundable tax will generally be refunded to a corporate Canadian Resident Holder at the rate of 38 1/3% of taxable dividends paid while it is a private corporation.
Minimum Tax
Capital gains realized and dividends received by a Canadian Resident Holder that is an individual or a trust, other than certain specified trusts, may give rise to minimum tax under the Canadian Tax Act. Such Canadian Resident Holders should consult their own advisors with respect to the application of minimum tax.
Non-Residents of Canada
The following section of this summary is generally applicable to a Holder who, for the purposes of the Canadian Tax Act, and at all relevant times: (i) has not been and will not be deemed to be resident in Canada; and (ii) does not use or hold the common shares or Warrants in, or in the course of, carrying on a business, or part of a business, in Canada, each a Non-Canadian Holder. Special rules, which are not discussed in this summary, may apply to a Non-Canadian Holder that is an insurer carrying on business in Canada and elsewhere or that is an “authorized foreign bank” as defined in the Canadian Tax Act. Such a Non-Canadian Holder should consult its own tax advisors.
Dividends
Dividends on the common shares paid or credited or deemed to be paid or credited to a Non-Canadian Holder will be subject to Canadian withholding tax at the rate of 25% on the gross amount of the dividend unless such rate is reduced by the terms of an applicable tax treaty. Under the Canada-United States Income Tax Convention (1980), or the Treaty, as amended, the rate of withholding tax on dividends paid or credited to a Non-Canadian Holder who is resident in the U.S. for purposes of the Treaty, is entitled to the full benefits under the Treaty and beneficially owns the dividend, or a U.S. Holder, is generally limited to 15% of the gross amount of the dividend (or 5% in the case of a U.S. Holder that is a corporation beneficially owning at least 10% of the Company’s voting shares). Not all persons who are residents of the U.S. for purposes of the Treaty will qualify for the benefits of the Treaty. Non-Canadian Holders that are resident in the U.S. are advised to consult their tax advisors in this regard. The rate of withholding tax on dividends is also reduced under other bilateral income tax treaties or conventions to which Canada is a signatory.
24
Expiry of Warrants
In the event of the expiry of an unexercised Warrant, a Non-Canadian Holder will be considered to have disposed of such Warrant for nil proceeds and will accordingly realize a capital loss equal to the Canadian Resident Holder’s adjusted cost base of such Warrant immediately before that time. For a description of the tax treatment of capital losses, see the discussion under “Non-Residents of Canada - Disposition of Warrants, and Common Shares”, below.
Exercise of Warrants
No gain or loss will be realized by a Non-Canadian Holder on the exercise of a Warrant. When a Warrant is exercised, the Non-Canadian Holder’s cost of the common shares acquired thereby will be equal to the adjusted cost base of the Warrant, immediately before that time, plus the amount paid on the exercise of the Warrant. For the purpose of computing the adjusted cost base of each common share acquired on the exercise of a Warrant, the cost of such common share must be averaged with the adjusted cost base to the Canadian Resident Holder of all other common shares held as capital property immediately before the exercise of the Warrant.
Dispositions of Common Shares and Warrants
A Non-Canadian Holder generally will not be subject to tax under the Canadian Tax Act in respect of a capital gain realized on the disposition or deemed disposition of a common share or Warrant nor will capital losses arising therefrom be recognized under the Canadian Tax Act, unless the common share or Warrant constitutes “taxable Canadian property” to the Non-Canadian Holder thereof for purposes of the Canadian Tax Act, and the gain is not exempt from Canadian federal income tax pursuant to the terms of an applicable tax treaty.
Generally the common shares or Warrants acquired pursuant to this offering will not be “taxable Canadian property” to a Non-Canadian Holder if the common shares are listed on a “designated stock exchange”, as defined in the Canadian Tax Act (which currently includes Nasdaq) at the time of disposition, unless at any time during the 60 month period immediately preceding the disposition the following two conditions are met concurrently: (i) the Non-Canadian Holder, persons with whom the Non-Canadian Holder did not deal at arm’s length, partnerships in which the Non-Canadian Holder or persons with whom the Non-Canadian Holder did not deal at arm’s length held a membership interest (either directly or indirectly through one or more partnerships), or the Non-Canadian Holder together with all such persons, owned 25% or more of the Company’s issued shares of any class or series of the Company’s shares; and (ii) more than 50% of the fair market value of such shares was derived directly or indirectly from one, or any combination of, real or immovable property situated in Canada, “Canadian resource properties” (as defined in the Canadian Tax Act), “timber resource properties” (as defined in the Canadian Tax Act) or an option, an interest or right in such property, whether or not such property exists. Notwithstanding the foregoing, a common share may otherwise be deemed to be taxable Canadian property to a Non-Canadian Holder for purposes of the Canadian Tax Act.
Provided that the common shares are listed on a “recognized stock exchange” (which currently includes Nasdaq), as defined in the Canadian Tax Act at the time of the disposition or deemed disposition of a common share or Warrant, a Non-Canadian Holder that disposes of a common share or Warrant that is taxable Canadian property will not be required to satisfy the obligations imposed under section 116 of the Canadian Tax Act and, as such, the purchaser of such shares or Warrants will not be required to withhold any amount on the purchase price paid. An exemption from such requirements may also be available in respect of such disposition if the common shares or Warrants are “treaty exempt property,” as defined in the Canadian Tax Act.
A Non-Canadian Holder’s capital gain (or capital loss) in respect of common shares or Warrants that constitute or are deemed to constitute taxable Canadian property (and are not “treaty-protected property” as defined in the Canadian Tax Act) will generally be computed and included in income in the manner described above under the subheadings “Residents of Canada—Dispositions of Common Shares or Warrants” and “Residents of Canada—Capital Gains and Capital Losses”.
Non-Canadian Holders whose common shares may be taxable Canadian property should consult their own tax advisors.
25
Material U.S. Federal Income Tax Considerations for U.S. Holders
The following is a general summary of certain material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from and relating to the exercise, disposition, and lapse of the Warrants and the acquisition, ownership, and disposition of the common shares received upon exercise of the Warrants (the “Warrant Shares”).
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder as a result of the acquisition of Warrant Shares pursuant to this prospectus. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including, without limitation, specific tax consequences to a U.S. Holder under an applicable income tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. This summary does not address the U.S. alternative minimum, U.S. federal net investment income, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences to U.S. Holders of the exercise, disposition, and lapse of the Warrants and acquisition, ownership, and disposition of Warrant Shares. In addition, except as specifically set forth below, this summary does not discuss applicable tax reporting requirements. Each U.S. Holder should consult its own tax advisors regarding the U.S. federal alternative minimum, U.S. federal net investment income, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the exercise, disposition, and lapse of the Warrants, and the acquisition, ownership and disposition of Warrant Shares.
No legal opinion from legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences described herein. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed) promulgated under the Code, published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions, that are applicable, and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied retroactively. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Warrant Shares acquired pursuant to this prospectus or Warrants described in this prospectus that is for U.S. federal income tax purposes:
| · | an individual that is a citizen or resident of the United States; |
| · | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia; |
| · | an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| · | a trust that (1) is subject to the primary supervision of a court within the U.S. and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
26
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including U.S. Holders that: (a) are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) are brokers or dealers in securities or currencies or U.S. Holders that are traders in securities that elect to apply a mark-to-market accounting method; (d) have a “functional currency” other than the U.S. dollar; (e) own Warrants or Warrant Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other integrated transaction; (f) acquired Warrants or Warrant Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) hold Warrants or Warrant Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); (h) are partnerships and other pass-through entities (and investors in such partnerships and entities); (i) are S corporations (and shareholders thereof); (j) are subject to special tax accounting rules with respect to the Warrants or Warrant Shares; (k) own, have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power or value of the Company’s outstanding shares; (l) are subject to taxing jurisdictions other than, or in addition to, the United States or otherwise hold Warrants or Warrant Shares in connection with a trade or business, permanent establishment, or fixed base outside the United States; (m) are U.S. expatriates or former long-term residents of the United States subject to Section 877 or 877A of the Code; or (n) are subject to the alternative minimum tax. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described immediately above, should consult their own tax advisors regarding the U.S. federal, U.S. federal net investment income, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the exercise, disposition, and lapse of the Warrants and the acquisition, ownership and disposition of Warrant Shares.
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds Warrants or Warrant Shares, the U.S. federal income tax consequences to such entity or arrangement and the owners of such entity or arrangement generally will depend on the activities of such entity or arrangement and the status of such owners. This summary does not address the tax consequences to any such entity or arrangement or owner. Owners of entities or arrangements that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisor regarding the U.S. federal income tax consequences arising from and relating to the exercise, disposition, and lapse of the Warrants and the acquisition, ownership, and disposition of Warrant Shares.
Passive Foreign Investment Company Rules
If the Company were to constitute a “passive foreign investment company” under the meaning of Section 1297 of the Code (a “PFIC”, as defined below) for any year during a U.S. Holder’s holding period then certain potentially adverse rules would affect the U.S. federal income tax consequences to a U.S. Holder as a result of the acquisition, ownership, and disposition of Warrant Shares.
The Company believes that it was classified as a PFIC during the taxable year ended December 31, 2022, and based on current business plans and financial expectations, the Company may be a PFIC for the taxable year ending December 31, 2023 or future taxable years. No opinion of legal counsel or ruling from the IRS concerning the status of the Company as a PFIC has been obtained or is currently planned to be requested. The determination of whether any corporation was, or will be, a PFIC for a tax year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. In addition, whether any corporation will be a PFIC for any tax year depends on the assets and income of such corporation over the course of each such tax year and, as a result, cannot be predicted with certainty as of the date of this document. Accordingly, there can be no assurance that the IRS will not challenge any PFIC determination made by the Company (or any subsidiary of the Company) concerning its PFIC status. Each U.S. Holder should consult its own tax advisors regarding the PFIC status of the Company and each subsidiary of the Company.
In any year in which the Company is classified as a PFIC, a U.S. Holder will be required to file an annual report with the IRS containing such information as Treasury Regulations and/or other IRS guidance may require. In addition to penalties, a failure to satisfy such reporting requirements may result in an extension of the time period during which the IRS can assess a tax. U.S. Holders should consult their own tax advisors regarding the requirements of filing such information returns under these rules, including the requirement to file an IRS Form 8621 annually.
The Company generally will be a PFIC if, for a tax year , (a) 75% or more of the gross income of the Company is passive income (the “PFIC income test”) or (b) 50% or more of the value of the assets of the Company either produce passive income or are held for the production of passive income, based on the quarterly average of the fair market value of such assets (the “PFIC asset test”). “Gross income” generally includes all sales revenues less the cost of goods sold, plus income from investments and from incidental or outside operations or sources, and “passive income” generally includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions.
27
For purposes of the PFIC income test and PFIC asset test described above, if the Company owns, directly or indirectly, 25% or more of the total value of the outstanding shares of another corporation, the Company will be treated as if it (a) held a proportionate share of the assets of such other corporation and (b) received directly a proportionate share of the income of such other corporation. In addition, for purposes of the PFIC income test and PFIC asset test described above, and assuming certain other requirements are met, “passive income” does not include certain interest, dividends, rents, or royalties that are received or accrued by the Company from certain “related persons” (as defined in Section 954(d)(3) of the Code) also organized in Canada, to the extent such items are properly allocable to the income of such related person that is not passive income.
Under certain attribution rules, if the Company is a PFIC, U.S. Holders will generally be deemed to own their proportionate share the Company’s direct or indirect equity interest in any company that is also a PFIC (a “Subsidiary PFIC”), and will generally be subject to U.S. federal income tax on their proportionate share of any (a) any “excess distributions,” as described below, on the stock of a Subsidiary PFIC and (b) a disposition or deemed disposition of the stock of a Subsidiary PFIC by the Company or another Subsidiary PFIC, both as if such U.S. Holders directly held the shares of such Subsidiary PFIC. In addition, U.S. Holders may be subject to U.S. federal income tax on any indirect gain realized on the stock of a Subsidiary PFIC on the sale or disposition of Common Shares. Accordingly, U.S. Holders should be aware that they could be subject to tax under the PFIC rules even if no distributions are received and no redemptions or other dispositions of Warrant Shares are made.
Default PFIC Rules Under Section 1291 of the Code
If the Company is a PFIC for any tax year during which a U.S. Holder owns Common Shares, the U.S. federal income tax consequences to such U.S. Holder of the acquisition, ownership, and disposition of Warrant Shares will depend on whether and when such U.S. Holder makes a “qualified electing fund” or “QEF” under Section 1295 of the Code (a “QEF Election”) or makes a mark-to-market election under Section 1296 of the Code (a “Mark-to-Market Election”). A U.S. Holder that does not make either a QEF Election or a Mark-to-Market Election will be referred to in this summary as a “Non-Electing U.S. Holder.”
A Non-Electing U.S. Holder will be subject to the rules of Section 1291 of the Code (as described below) with respect to (a) any gain recognized on the sale or other taxable disposition of Warrant Shares and (b) any excess distribution received on the Warrant Shares. A distribution generally will be an “excess distribution” to the extent that such distribution (together with all other distributions received in the current tax year) exceeds 125% of the average distributions received during the three preceding tax years (or during a U.S. Holder’s holding period for the Warrant Shares, if shorter).
Under Section 1291 of the Code, any gain recognized on the sale or other taxable disposition of Warrant Shares (including an indirect disposition of the stock of a Subsidiary PFIC), and any “excess distribution” received on such Warrant Shares or with respect to the stock of a Subsidiary PFIC, must be ratably allocated to each day in a Non-Electing U.S. Holder’s holding period for the respective Warrant Shares. The amount of any such gain or excess distribution allocated to the tax year of disposition or distribution of the excess distribution and to years before the entity became a PFIC, if any, would be taxed as ordinary income (and not eligible for certain preferred rates). The amounts allocated to any other tax year would be subject to U.S. federal income tax at the highest tax rate applicable to ordinary income in each such year, and an interest charge would be imposed on the tax liability for each such year, calculated as if such tax liability had been due in each such year. A Non-Electing U.S. Holder that is not a corporation must treat any such interest paid as “personal interest,” which is not deductible.
If the Company is a PFIC for any tax year during which a Non-Electing U.S. Holder holds Warrant Shares or Warrants, the Company will continue to be treated as a PFIC with respect to such Non-Electing U.S. Holder, regardless of whether the Company ceases to be a PFIC in one or more subsequent tax years. A Non-Electing U.S. Holder may terminate this deemed PFIC status by electing to recognize gain (which will be taxed under the rules of Section 1291 of the Code discussed above) but not loss, as if such Warrant Shares were sold on the last day of the last tax year for which the Company was a PFIC. No such election, however, may be made with respect to the Warrants.
Under proposed Treasury Regulations, if a U.S. holder has an option, warrant, or other right to acquire stock of a PFIC (such as the Warrants), such option, warrant or right is considered to be PFIC stock subject to the default rules of Section 1291 of the Code. Under rules described below, the holding period for the Warrant Shares will begin on the date a U.S. Holder acquires the Warrants. This will impact the availability of the QEF Election and Mark-to-Market Election with respect to the Warrant Shares.
28
QEF Election
As discussed above, under proposed Treasury Regulations, if a U.S. holder has an option, warrant or other right to acquire stock of a PFIC (such as the Warrants), such option, warrant or right is considered to be PFIC stock subject to the default rules of Section 1291 of the Code. However, a U.S. Holder of an option, warrant or other right to acquire stock of a PFIC may not make a QEF Election that will apply to the option, warrant or other right to acquire PFIC stock. In addition, under proposed Treasury Regulations, if a U.S. Holder holds an option, warrant or other right to acquire stock of a PFIC, the holding period with respect to shares of stock of the PFIC acquired upon exercise of such option, warrant or other right will include the period that the option, warrant or other right was held.
Consequently, under the proposed Treasury Regulations, if a U.S. Holder of Common Shares makes a QEF Election, such election generally will not be treated as a timely QEF Election with respect to Warrant Shares and the rules of Section 1291 of the Code discussed above will continue to apply with respect to such U.S. Holder’s Warrant Shares. However, a U.S. Holder of Warrant Shares should be eligible to make a timely QEF Election if such U.S. Holder elects in the tax year in which such Warrant Shares are received to recognize gain (which will be taxed under the rules of Section 1291 of the Code discussed above) as if such Warrant Shares were sold for fair market value on the date such U.S. Holder acquired them by exercising the corresponding Warrant. In addition, gain recognized on the sale or other taxable disposition (other than by exercise) of the Warrants by a U.S. Holder will be subject to the rules of Section 1291 of the Code discussed above. Each U.S. Holder should consult its own tax advisors regarding the application of the PFIC rules to the Warrants and Warrant Shares.
A U.S. Holder that makes a timely and effective QEF Election under the rules set forth in the preceding paragraph generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to its Warrant Shares. However, a U.S. Holder that makes a timely and effective QEF Election will be subject to U.S. federal income tax on such U.S. Holder’s pro rata share of (a) the Company’s net capital gain, which will be taxed as long-term capital gain to such U.S. Holder, and (b) the Company’s ordinary earnings, which will be taxed as ordinary income to such U.S. Holder. Generally, “net capital gain” is the excess of (a) net long-term capital gain over (b) net short-term capital loss, and “ordinary earnings” are the excess of (a) “earnings and profits” over (b) net capital gain. A U.S. Holder that makes a QEF Election will be subject to U.S. federal income tax on such amounts for each tax year in which the Company is a PFIC, regardless of whether such amounts are actually distributed to such U.S. Holder by the Company. However, for any tax year in which the Company is a PFIC and has no net income or gain, U.S. Holders that have made a QEF Election would not have any income inclusions as a result of the QEF Election. If a U.S. Holder that made a QEF Election has an income inclusion, such a U.S. Holder may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a corporation, any such interest paid will be treated as “personal interest,” which is not deductible.
A U.S. Holder that makes a timely and effective QEF Election as described above generally (a) may receive a tax-free distribution from the Company to the extent that such distribution represents “earnings and profits” of the Company that were previously included in income by the U.S. Holder because of such QEF Election and (b) will adjust such U.S. Holder’s tax basis in the Warrant Shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, a U.S. Holder that makes a QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of Warrant Shares.
A QEF Election will apply to the tax year for which such QEF Election is timely made and to all subsequent tax years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent tax year, the Company ceases to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those tax years in which the Company is not a PFIC. Accordingly, if the Company becomes a PFIC in another subsequent tax year, the QEF Election will be effective and the U.S. Holder will be subject to the QEF rules described above during any subsequent tax year in which the Company qualifies as a PFIC. U.S. Holders should be aware that if the Company determines that it is a PFIC for this year or any future taxable year, it can make no assurances that it would provide the information necessary for U.S. Holders to make a QEF Election. Thus, U.S. Holders may not be able to make a QEF Election with respect to their Warrant Shares.
A U.S. Holder makes a QEF Election by attaching a completed IRS Form 8621, including a PFIC Annual Information Statement, to a timely filed U.S. federal income tax return. However, if the Company does not provide the required information with regard to the Company or any of its Subsidiary PFICs, U.S. Holders will not be able to make a QEF Election for such entity and will continue to be subject to the rules of Section 1291 of the Code discussed above that apply to Non-Electing U.S. Holders with respect to the taxation of gains and excess distributions.
29
Mark-to-Market Election
A U.S. Holder may make a Mark-to-Market Election with respect to Warrant Shares only if the Warrant Shares are marketable stock. The Warrant Shares generally will be “marketable stock” if the Warrant Shares are regularly traded on (a) a national securities exchange that is registered with the SEC, (b) the national market system established pursuant to Section 11A of the U.S. Exchange Act or (c) a foreign securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such foreign exchange has trading volume, listing, financial disclosure, surveillance requirements, and meets other requirements and the laws of the country in which such foreign exchange is located, together with the rules of such foreign exchange, ensure that such requirements are actually enforced and (ii) the rules of such foreign exchange ensure active trading of listed stocks. If such stock is traded on such a qualified exchange or other market, such stock generally will be considered “regularly traded” for any calendar year during which such stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. Provided that the Warrant Shares are “regularly traded” as described in the preceding sentence, the Warrant Shares are expected to be marketable stock. However, Each U.S. Holder should consult its own tax advisor in this matter.
A U.S. Holder that makes a Mark-to-Market Election with respect to its Warrant Shares generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to such Warrant Shares. However, if a U.S. Holder does not make a Mark-to-Market Election beginning in the first tax year of such U.S. Holder’s holding period for the Warrant Shares for which the Company is a PFIC and such U.S. Holder has not made a timely QEF Election, the rules of Section 1291 of the Code discussed above will apply to certain dispositions of, and distributions on, the Warrant Shares.
Any Mark-to-Market Election made by a U.S. Holder for its Common Shares will also apply to such U.S. Holder’s Warrant Shares. As a result, if a Mark-to-Market Election has been made by a U.S. Holder with respect to its Common Shares, any Warrant Shares received will automatically be marked-to-market in the year of exercise. Because, under the proposed Treasury Regulations, a U.S. Holder’s holding period for Warrant Shares includes the period during which such U.S. Holder held the Warrants, a U.S. Holder will be treated as making a Mark-to-Market Election with respect to its Warrant Shares after the beginning of such U.S. Holder’s holding period for the Warrant Shares unless the Warrant Shares are acquired in the same tax year as the year in which the U.S. Holder acquired its Warrants. Consequently, the default rules under Section 1291 described above generally will apply to the mark-to-market gain realized in the tax year in which Warrant Shares are received. However, the general mark-to-market rules will apply to subsequent tax years.
A U.S. Holder that makes a Mark-to-Market Election will include in ordinary income, for each tax year in which the Company is a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the Warrant Shares, as of the close of such tax year over (b) such U.S. Holder’s adjusted tax basis in the Warrant Shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an amount equal to the excess, if any, of (i) such U.S. Holder’s adjusted tax basis in the Warrant Shares, over (ii) the fair market value of such Warrant Shares (but only to the extent of the net amount of previously included income as a result of the Mark-to-Market Election for prior tax years).
A U.S. Holder that makes a Mark-to-Market Election generally also will adjust such U.S. Holder’s tax basis in the Warrant Shares to reflect the amount included in gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition of Warrant Shares, a U.S. Holder that makes a Mark-to-Market Election will recognize ordinary income or ordinary loss (not to exceed the excess, if any, of (a) the amount included in ordinary income because of such Mark-to-Market Election for prior tax years over (b) the amount allowed as a deduction because of such Mark-to-Market Election for prior tax years).
A U.S. Holder makes a Mark-to-Market Election by attaching a completed IRS Form 8621 to a timely filed U.S. federal income tax return. Mark-to-Market Election applies to the tax year in which such Mark-to-Market Election is made and to each subsequent tax year, unless the Warrant Shares cease to be “marketable stock” or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisors regarding the availability of, and procedure for making, a Mark-to-Market Election.
Although a U.S. Holder may be eligible to make a Mark-to-Market Election with respect to the Warrant Shares, no such election may be made with respect to the stock of any Subsidiary PFIC that a U.S. Holder is treated as owning because such stock is not marketable. Hence, the Mark-to-Market Election will not be effective to avoid the application of the default rules of Section 1291 of the Code described above with respect to deemed dispositions of Subsidiary PFIC stock or excess distributions from a Subsidiary PFIC to its shareholder.
Other PFIC Rules
Under Section 1291(f) of the Code, the IRS has issued proposed Treasury Regulations that, subject to certain exceptions, would cause a U.S. Holder that had not made a timely QEF Election to recognize gain (but not loss) upon certain transfers of Warrant Shares that would otherwise be tax-deferred (e.g., gifts and exchanges pursuant to corporate reorganizations). However, the specific U.S. federal income tax consequences to a U.S. Holder may vary based on the manner in which Warrants or Warrant Shares are transferred.
30
If finalized in their current form, the proposed Treasury Regulations applicable to PFICs would be effective for transactions occurring on or after April 1, 1992. Because the proposed Treasury Regulations have not yet been adopted in final form, they are not currently effective, and there is no assurance that they will be adopted in the form and with the effective date proposed. Nevertheless, the IRS has announced that, in the absence of final Treasury Regulations, taxpayers may apply reasonable interpretations of the Code provisions applicable to PFICs and that it considers the rules set forth in the proposed Treasury Regulations to be reasonable interpretations of those Code provisions. The PFIC rules are complex, and the implementation of certain aspects of the PFIC rules requires the issuance of Treasury Regulations which in many instances have not been promulgated and which, when promulgated, may have retroactive effect. U.S. Holders should consult their own tax advisors about the potential applicability of the proposed Treasury Regulations.
Certain additional adverse rules may apply with respect to a U.S. Holder if the Company is a PFIC, regardless of whether such U.S. Holder makes a QEF Election. For example, under Section 1298(b)(6) of the Code, a U.S. Holder that uses Warrants or Warrant Shares as security for a loan will, except as may be provided in Treasury Regulations, be treated as having made a taxable disposition of such Warrants or Warrant Shares.
In addition, a U.S. Holder who acquires Warrants or Warrant Shares from a decedent will not receive a “step up” in tax basis of such Warrants or Warrant Shares to fair market value.
Special rules also apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, foreign taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complicated, and a U.S. Holder should consult with its own tax advisors regarding the availability of the foreign tax credit with respect to distributions by a PFIC.
The PFIC rules are complex, and each U.S. Holder should consult its own tax advisors regarding the PFIC rules and how the PFIC rules may affect the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Warrants and Warrant Shares.
U.S. Federal Income Tax Consequences of the Ownership, Exercise and Disposition of Warrants
The following discussion describes the general rules applicable to the ownership, exercise and disposition of the Warrants but is subject in its entirety to the special rules described above under the heading “Passive Foreign Investment Company Rules.”
Exercise of Warrants
A U.S. Holder should not recognize gain or loss on the exercise of a Warrant and related receipt of a Warrant Share (unless cash is received in lieu of the issuance of a fractional Warrant Share). A U.S. Holder’s initial tax basis in the Warrant Share received on the exercise of a Warrant should be equal to the sum of (a) such U.S. Holder’s tax basis in such Warrant plus (b) the exercise price paid by such U.S. Holder on the exercise of such Warrant. It is unclear whether a U.S. Holder’s holding period for the Warrant Share received on the exercise of a Warrant would commence on the date of exercise of the Warrant or the day following the date of exercise of the Warrant. If the Company is a PFIC, a U.S. Holder’s holding period for the Warrant Share for PFIC purposes only will begin on the date on which such U.S. Holder acquired its Warrants.
In certain limited circumstances, a U.S. Holder may be permitted to undertake a cashless exercise of Warrants into Warrant Shares. The U.S. federal income tax treatment of a cashless exercise of Warrants into Warrant Shares is unclear, and the tax consequences of a cashless exercise could differ from the consequences upon the exercise of a Warrant described in the preceding paragraph. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of Warrants.
Disposition of Warrants
A U.S. Holder will recognize gain or loss on the sale or other taxable disposition of a Warrant in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received and (b) such U.S. Holder’s tax basis in the Warrant sold or otherwise disposed of. Subject to the PFIC rules discussed above, any such gain or loss generally will be a capital gain or loss, which will be long-term capital gain or loss if the Warrant is held for more than one year. Deductions for capital losses are subject to complex limitations under the Code.
Expiration of Warrants Without Exercise
Upon the lapse or expiration of a Warrant, a U.S. Holder will recognize a loss in an amount equal to such U.S. Holder’s tax basis in the Warrant. Any such loss generally will be a capital loss and will be long-term capital loss if the Warrants are held for more than one year. Deductions for capital losses are subject to complex limitations under the Code.
31
Certain Adjustments to the Warrants
Under Section 305 of the Code, an adjustment to the number of Warrant Shares that will be issued on the exercise of the Warrants, or an adjustment to the exercise price of the Warrants, may be treated as a constructive distribution to a U.S. Holder of the Warrants if, and to the extent that, such adjustment has the effect of increasing such U.S. Holder’s proportionate interest in the “earnings and profits” or the Company’s assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to the shareholders). Adjustments to the exercise price of Warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the Warrants should generally not be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property. (See more detailed discussion of the rules applicable to distributions made by the Company at “Distributions on Warrant Shares” below).
General Rules Applicable to U.S. Federal Income Tax Consequences of the Acquisition, Ownership, and Disposition of Warrant Shares
The following discussion describes the general rules applicable to the acquisition, ownership and disposition of the Warrant Shares but is subject in its entirety to the special rules described above under the heading “Passive Foreign Investment Company Rules.”
Distributions on Warrant Shares
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to a Warrant Share (as well as any constructive distribution on a Warrant as described above) will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of the Company’s current and accumulated “earnings and profits”, as computed for U.S. federal income tax purposes. A dividend generally will be taxed to a U.S. Holder at ordinary income tax rates if the Company is a PFIC for the tax year of such distribution or the preceding tax year. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Company, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Warrant Shares and thereafter as gain from the sale or exchange of such Warrant Shares (see “Sale or Other Taxable Disposition of Warrant Shares” below). However, the Company may not maintain the calculations of earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder may be required to assume that any distribution by the Company with respect to the Warrant Shares will constitute ordinary dividend income. Dividends received on Warrant Shares by corporate U.S. Holders generally will not be eligible for the “dividends received deduction.” Subject to applicable limitations and provided the Company is eligible for the benefits of the Canada-U.S. Tax Convention, or the Common Shares are readily tradable on a United States securities market, dividends paid by the Company to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends, provided certain holding period and other conditions are satisfied, including that the Company not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisors regarding the application of such rules.
Sale or Other Taxable Disposition of Warrant Shares
Upon the sale or other taxable disposition of Warrant Shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the U.S. dollar value of cash received plus the fair market value of any property received and such U.S. Holder’s tax basis in such Warrant Shares sold or otherwise disposed of. A U.S. Holder’s tax basis in Warrant Shares generally will be such holder’s U.S. dollar cost for such Warrant Shares. Gain or loss recognized on such sale or other disposition generally will be long-term capital gain or loss if, at the time of the sale or other disposition, the Warrant Shares have been held for more than one year.
Preferential tax rates currently apply to long-term capital gain of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gain of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code.
32
Additional Tax Considerations
Receipt of Foreign Currency
The amount of any distribution paid to a U.S. Holder in foreign currency or on the sale, exchange or other taxable disposition of Warrants or Warrant Shares generally will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt (regardless of whether such foreign currency is converted into U.S. dollars at that time). If the foreign currency received is not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a tax basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who converts or otherwise disposes of the foreign currency after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Warrant Shares (or with respect to any constructive dividend on the Warrants) generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid or accrued (whether directly or through withholding) by a U.S. Holder during a year. The foreign tax credit rules are complex and involve the application of rules that depend on a U.S. Holder’s particular circumstances. Accordingly, each U.S. Holder should consult its own U.S. tax advisors regarding the foreign tax credit rules.
Backup Withholding and Information Reporting
Under U.S. federal income tax law certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals that are U.S. Holders that hold certain specified foreign financial assets in excess of certain thresholds. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U.S. Holders may be subject to these reporting requirements unless their Warrants and Warrant Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns, including the requirement to file an IRS Form 8938.
Payments made within the U.S., or by a U.S. payor or U.S. middleman, of dividends on, and proceeds arising from the sale or other taxable disposition of the Warrants and Warrant Shares will generally be subject to information reporting and backup withholding tax, currently at the rate of 24%, if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that it has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax. However, certain exempt persons, generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding tax rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner.
The discussion of reporting requirements set forth above is not intended to constitute a complete description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax and, under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. Each U.S. Holder should consult its own tax advisors regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR OWN PARTICULAR CIRCUMSTANCES.
We will deliver common shares upon the exercise of the warrants. The Warrants contain instructions for exercise. We will deliver common shares in the manner described above in the section titled “Description of Warrants”. We do not know if or when the Warrants will be exercised. We also do not know whether any of the common shares acquired upon exercise will be sold.
33
EXPENSES RELATED TO THIS OFFERING
The following table sets forth the costs and expenses payable by us in connection with the offer and sale of our common shares in this offering. All amounts listed below are estimates.
| Itemized expense | Amount | |||
| Printing and engraving expenses | 20,000 | |||
| Transfer agent and registrar fees | 10,000 | |||
| Legal fees and expenses | 30,000 | |||
| Accounting fees and expenses | 30,000 | |||
| Total | 90,000 |
The validity of the securities being offered by this prospectus and other legal matters concerning this offering relating to Canadian law will be passed upon for us by Fasken Martineau DuMoulin LLP. Certain legal matters in connection with this offering relating to U.S. law will be passed upon for us by Troutman Pepper Hamilton Sanders LLP.
The consolidated financial statements of XORTX as of and for the year ended December 31, 2022, have been audited by Smythe LLP, independent registered public accounting firm, as set forth in their report thereon. Smythe LLP is independent with respect to us within the meaning of the Rules of Professional Conduct of the Institute of Chartered Professional Accountants of British Columbia and under all relevant U.S. professional and regulatory standards, including Public Company Accounting Oversight Board Rule 3520. We have included our financial statements in this prospectus and in this registration statement in reliance on the report of Smythe LLP given on their authority as experts in accounting and auditing.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or person controlling the registrant in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We file reports and other information with the securities commissions and similar regulatory authorities in the provinces of Canada (collectively, the “Commissions”). These reports and information are available to the public free of charge on SEDAR at www.sedar.com.
34
We are subject to the information requirements of the Exchange Act relating to foreign private issuers and applicable Canadian securities legislation and, in accordance therewith, file reports and other information with the SEC and securities regulatory authorities in Canada The SEC maintains an internet website at www.sec.gov, from which you can electronically access the documents we have filed with the SEC’s Electronic Data Gathering and Retrieval system at www.sec.gov, including registration statement of which this prospectus forms a part and its exhibits.
Readers should rely only on information contained or incorporated by reference in this prospectus and any applicable Prospectus Supplement. We have not authorized anyone to provide the reader with different information. We are not making an offer of the Securities in any jurisdiction where the offer is not permitted. Readers should not assume that the information contained in this Prospectus is accurate as of any date other than the date on the front of this Prospectus, unless otherwise noted herein or as required by law. It should be assumed that the information appearing in this Prospectus and the documents incorporated herein by reference are accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have changed since those dates.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” the information we file with the SEC. This means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is considered to be part of this prospectus.
We incorporate by reference the documents listed below:
| · | our Annual Report on Form 20-F for the fiscal year ended December 31, 2022 filed with the SEC on April 28, 2023; |
| · | our Reports on Form 6-K furnished to the SEC on May 4, 2023, May 16, 2023, May 24, 2023, May 30, 2023, and June 30, 2023; |
| · | the description of the securities contained in our Amendment No. 1 to the registration statement on Form 8-A filed with the SEC on October 4, 2021 (File No. 001-40858) pursuant to Section 12 of the Exchange Act, together with all amendments and reports filed for the purpose of updating that description. |
We will provide, free of charge upon written or oral request, to each person to whom this prospectus is delivered, including any beneficial owner of the securities, a copy of any or all of the information that has been incorporated by reference into this prospectus, but which has not been delivered with the prospectus. The information contained on or linked to or from our website is not incorporated by reference into this prospectus and should not be considered part of this prospectus. Requests for such information should be made to us at the following address:
3710 – 33rd Street NW Calgary, Alberta, Canada, T2L 2M1
1 (403) 455-7727
info@xortx.com
You should assume that the information appearing in this prospectus, as well as the information we previously filed with the SEC and incorporated by reference, is accurate as of the dates on the front cover of those documents only. Our business, financial condition and results of operations and prospects may have changed since those dates.
35

5,000,000 Common Shares Issuable upon
Exercise of Warrants
PROSPECTUS
, 2023
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers
XORTX is subject to the provisions of Part 5, Division 5 of the Business Corporations Act (British Columbia) (the “BCBCA”). Under Section 160 of the BCBCA, XORTX may, subject to Section 163 of the BCBCA do one or both of the following:
| (a) | indemnify an eligible party against all eligible penalties to which the eligible party is or may be liable; or |
| (b) | after final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by an eligible party in respect of that proceeding, |
where:
| (i) | “eligible party” means, in relation to XORTX, means an individual who |
| i. | is or was a director or officer of XORTX, |
| ii. | is or was a director or officer of another corporation |
| 1. | at a time when the corporation is or was an affiliate of XORTX, or |
| 2. | at the request of XORTX, or |
| iii. | at the request of XORTX, is or was, or holds or held a position equivalent to that of, a director or officer of a partnership, trust, joint venture or other unincorporated entity, and includes, except in the definition of “eligible proceeding” and except in sections 163(1)(c) and (d) and 165 of the BCBCA, the heirs and personal or other legal representatives of that individual; |
| (ii) | “eligible penalty” means a judgment, penalty or fine awarded or imposed in, or an amount paid in settlement of, an eligible proceeding, |
| (iii) | “eligible proceeding” means a proceeding in which an eligible party or any of the heirs and personal or other legal representatives of the eligible party, by reason of the eligible party being or having been a director or officer of, or holding or having held a position equivalent to that of a director or officer of, XORTX or an associated corporation |
| i. | is or may be joined as a party, or |
| ii. | is or may be liable for or in respect of a judgment, penalty or fine in, or expenses related to, the proceeding, |
| (iv) | “expenses” includes costs, charges and expenses, including legal and other fees, but does not include judgments, penalties, fines or amounts paid in settlement of a proceeding, and |
| (v) | “proceeding” includes any legal proceeding or investigative action, whether current, threatened, pending or completed. |
Under Section 161 of the BCBCA, and subject to Section 163 of the BCBCA, XORTX must, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by an eligible party in respect of that proceeding if the eligible party (a) has not been reimbursed for those expenses and (b) is wholly successful, on the merits or otherwise, in the outcome of the proceeding or is substantially successful on the merits in the outcome of the proceeding.
II-1
Under Section 162 of the BCBCA, and subject to Section 163 of the BCBCA, XORTX may pay, as they are incurred in advance of the final disposition of an eligible proceeding, the expenses actually and reasonably incurred by an eligible party in respect of the proceeding, provided that XORTX must not make such payments unless it first receives from the eligible party a written undertaking that, if it is ultimately determined that the payment of expenses is prohibited under Section 163 of the BCBCA, the eligible party will repay the amounts advanced.
Under Section 163 of the BCBCA, XORTX must not indemnify an eligible party against eligible penalties to which the eligible party is or may be liable or pay the expenses of an eligible party under Sections 160(b), 161 or 162 of the BCBCA, as the case may be, if any of the following circumstances apply:
| (a) | if the indemnity or payment is made under an earlier agreement to indemnify or pay expenses and, at the time that the agreement to indemnify or pay expenses was made, XORTX was prohibited from giving the indemnity or paying the expenses by XORTX’s memorandum or Articles; |
| (b) | if the indemnity or payment is made otherwise than under an earlier agreement to indemnify or pay expenses and, at the time that the indemnity or payment is made, XORTX is prohibited from giving the indemnity or paying the expenses by XORTX’s memorandum or Articles; |
| (c) | if, in relation to the subject matter of the eligible proceeding, the eligible party did not act honestly and in good faith with a view to the best interests of XORTX or the associated corporation, as the case may be; or |
| (d) | in the case of an eligible proceeding other than a civil proceeding, if the eligible party did not have reasonable grounds for believing that the eligible party’s conduct in respect of which the proceeding was brought was lawful. |
If an eligible proceeding is brought against an eligible party by or on behalf of XORTX or by or on behalf of an associated corporation, XORTX must not either (a) indemnify the eligible party under Section 160(a) of the BCBCA against eligible penalties to which the eligible party is or may be liable in respect of the proceeding, or pay the expenses of the eligible party under Sections 160(b), 161 or 162 of the BCBCA, as the case may be, in respect of the proceeding.
Under Section 164 of the BCBCA, despite any other provision of Part 5, Division 5 of the BCBCA and whether or not payment of expenses or indemnification has been sought, authorized or declined under Part 5, Division 5 of the BCBCA, on application of XORTX or an eligible party, the court may do one or more of the following:
| (a) | order XORTX to indemnify an eligible party against any liability incurred by the eligible party in respect of an eligible proceeding; |
| (b) | order XORTX to pay some or all of the expenses incurred by an eligible party in respect of an eligible proceeding; |
| (c) | order the enforcement of, or any payment under, an agreement of indemnification entered into by XORTX; |
| (d) | order XORTX to pay some or all of the expenses actually and reasonably incurred by any person in obtaining an order under Section 164 of the BCBCA; or |
| (e) | make any other order the court considers appropriate. |
II-2
Section 165 of the BCBCA provides that XORTX may purchase and maintain insurance for the benefit of an eligible party or the heirs and personal or other legal representatives of the eligible party against any liability that may be incurred by reason of the eligible party being or having been a director or officer of, or holding or having held a position equivalent to that of a director or officer of, XORTX or an associated corporation.
Under XORTX’s articles, and subject to the BCBCA, XORTX must indemnify a director, former director or alternate director and his or her heirs and legal personal representatives against all eligible penalties to which such person is or may be liable, and XORTX must, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by such person in respect of that proceeding. Each director and alternate director is deemed to have contracted with XORTX on the terms of the indemnity contained in XORTX’s articles.
Under XORTX’s articles, and subject to the BCBCA, XORTX may agree to indemnify and may indemnify any person (including an eligible party). XORTX has not entered into indemnity agreements with its directors and officers.
Pursuant to XORTX’s articles, the failure of a director, alternate director or officer of XORTX to comply with the BCBCA or XORTX’s articles does not invalidate any indemnity to which he or she is entitled under XORTX’s articles.
Under XORTX’s articles, XORTX may purchase and maintain insurance for the benefit of any person (or his or her heirs or legal personal representatives) who:
| · | is or was a director, alternate director, officer, employee or agent of XORTX; |
| · | is or was a director, alternate director, officer, employee or agent of another corporation at a time when such corporation is or was an affiliate of XORTX; |
| · | at XORTX’s request, is or was, a director, alternate director, officer, employee or agent of a corporation or of a partnership, trust, joint venture or other unincorporated entity; or |
| · | at XORTX’s request, holds or held a position equivalent to that of, a director, alternate director or officer of a partnership, trust, joint venture or other unincorporated entity, |
against any liability incurred by him or her as a director, alternate director, officer, employee or agent or person who holds or held such equivalent position.
XORTX maintains directors’ and officers’ liability insurance which insures directors and officers for losses as a result of claims against the directors and officers of XORTX in their capacity as directors and officers.
At present, we are not aware of any pending or threatened litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification would be required or permitted.
Item 7. Recent Sales of Unregistered Securities
Set forth below is information regarding all securities issued by XORTX without registration under the Securities Act during the past three years after giving effect to the Company’s 2021 share consolidation that took place in 2021. The information presented below does not give effect to XORTX’s corporate reorganization as described in the prospectus forming part of this Registration Statement. XORTX believes that each of such issuances was exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, Rule 701 and/or Regulation S under the Securities Act. No underwriter or underwriting discount or commission was involved in any of the transactions set forth in this Item 7.
II-3
Common Share Issuances
| · | On February 28, 2020, we issued 1,555,314 of our common shares in a private placement, at a price of CAD$1.64 per share, for an aggregate offering price of CAD$2,556,320. |
| · | On February 9, 2021, the Company issued 2,085,687 units in a private placement offering at a subscription price of CAD$2.94 per unit for gross proceeds of CAD$6,121,572. Each unit comprised one Common Share of the Company and one common share purchase warrant (a “Private Placement Warrant”). Each Private Placement Warrant entitles the holder, on exercise, to purchase one additional Common Share in the capital of the Company, at a price of CAD$4.70, for a period of 5 years from the issuance of the units provided, however, that, if, at any time following the expiry of the statutory four month hold period, the closing price of the Common Shares on the TSXV is greater than CAD$14.09 for 10 or more consecutive trading days, the Private Placement Warrants will be accelerated upon notice and the Private Placement Warrants will expire on the 30th calendar day following the date of such notice. In addition, the Private Placement Warrants are also subject to typical anti-dilution provisions and a ratchet provision that provides for an adjustment in the exercise price should the Company issue or sell Common Shares or securities convertible into Common Shares at a price (or conversion price, as applicable) less than the exercise price such that the exercise price shall be amended to match such lower price. In connection with the February 9, 2021, private placement, the Company paid CAD$171,085 in cash commissions and issued 58,291 finder’s warrants. Each finder’s warrant is exercisable into one Common Share at a price of CAD$4.70 and having the same expiry, acceleration and anti-dilution provisions as the Private Placement Warrants included in the private placement. |
| · | Since January 1, 2019 through the date of this prospectus, we have issued 651,583 of our common shares pursuant to the exercise of various warrants, with exercise prices ranging from CAD$1.64 to CAD$4.70 per share, for aggregate consideration of CAD$2,430,083. |
| · | On February 1, March 1, and March 31, 2021, we issued an aggregate of 25,553 of our common shares at a price of CAD$2.94 per share, in exchange for services performed. |
| · | On October 15, 2021 and November 8, 2021, we issued 3,261,000 of our common shares in connection with the underwritten US IPO Offering (the “US IPO Offering”). 2,906,000 common shares were issued at closing on October 15, 2021 and 355,000 were issued in connection with a partial over-allotment exercise on November 8, 2021) at $4.13, with each unit consisting of one common share and one warrant (the “IPO Common Share Purchase Warrant”) for aggregate gross proceeds of $13,467,930. A.G.P./Alliance Global Partners acted as the representative of the underwriter and the sole book-running manager for the US IPO Offering. The common shares underlying the IPO Common Share Purchase Warrants were registered in this prospectus. |
| · | On October 7, 2022, the Company closed an underwritten public offering of: (i) 1,400,000 common share units (“October 2022 Common Share Units”), with each October 2022 Common Share Unit consisting of one common share, no par value, and one warrant (“Warrants”) to purchase one common share at a public offering price of $1.00 per Common Share Unit, and (ii) 3,600,000 pre-funded warrant units (“October 2022 Pre-Funded Units” and together with the Common Share Units, the “Units”), with each October 2022 Pre-Funded Unit consisting of one pre-funded warrant (“October 2022 Pre-Funded Warrant”) to purchase one common share and one Warrant to purchase one common share at a public offering price of $0.9999 per October 2022 Pre-Funded Unit, for aggregate gross proceeds of $4,999,640, prior to deducting underwriting discounts and other offering expenses and excluding any exercise of the underwriters’ option to purchase any additional securities as described herein. The common shares and Warrants contained in the Common Share Units and the October 2022 Pre-Funded Warrants and Warrants contained in the October 2022 Pre-Funded Units were immediately separable upon issuance. The Warrants have an initial exercise price of $1.22 per share, are immediately exercisable, and may be exercised for five years from the date of issuance. The October 2022 Pre-Funded Warrants had an exercise price of $0.0001 per share, were immediately exercisable, and terminated once exercised in full. As of the date of this Annual Report, all 3,600,000 October 2022 Pre-Funded Warrants have been exercised. In addition, the Company granted the underwriters of the October 2022 Offering a 45-day option to purchase up to an additional 750,000 common shares and/or warrants (“October 2022 Compensation Warrants”) to purchase up to an additional 750,000 common shares at the public offering price less the underwriting discounts. |
II-4
Further to an investment in connection with the October 2022 Offering, the Company entered into an agreement, approved by the TSXV, to reduce the exercise price of certain outstanding US IPO Common Share Purchase Warrants to purchase up to 910,000 shares of common stock issued in the US IPO Offering (the “Amended IPO Common Share Purchase Warrants”) and held by certain investors in the October 2022 Offering from $4.77 per share to $1.17 per share, effective upon the closing of the October 2022 Offering. All other terms of the Certain Prior US IPO Warrants remained the same.
| · | On December 29, 2022, we issued 641,000 of our common shares pursuant to the exercise of Pre-Funded Warrants, with an exercise price of $0.0001, for $64.00. |
| · | On January 19, 2023, we issued 2,959,000 of our common shares pursuant to the exercise of Pre-Funded Warrants, with exercise price of $0.0001, for $296.00. |
Stock Option Grants
| · | Since January 1, 2019, we have granted our employees, consultants and advisors options to purchase an aggregate of 1,167,095 options to acquire common shares under our equity compensation plans at exercise prices ranging from CAD$1.60 to CAD$5.87 per share. |
Warrants
| · | On February 28, 2020, we issued warrants to purchase an aggregate of 1,567,213 common shares for exercise prices ranging between CAD$1.64 to CAD$2.94 per share, in connection with the common share issuance of the same date referenced above. As of the date of this registration statement, all of the warrants have either been exercised or have expired. |
| · | On February 9, 2021, we issued Private Placement Warrants to purchase an aggregate of 2,144,005 common shares for an exercise price of CAD$4.70 per share, in connection with the common share issuance of the same date referenced above. As of the date of this registration statement, none of the Private Placement Warrants have been exercised. |
| · | On October 15, 2021, we issued IPO Common Share Purchase Warrants to purchase an aggregate of 3,341,900 common shares for an exercise price of $4.77 at the October 15, 2021 per share, in connection with the underwritten US IPO Offering. We issued 2,906,000 IPO Common Share Purchase Warrants in connection with the U.S. IPO Offering units and an additional 435,900 Common Share Purchase Warrants in connection with A.G.P./Alliance Global Partners’ exercise of its option for the purchase of up to 435,900 additional Common Share Purchase Warrants that same day. |
| · | On October 7, 2022, we issued 3,600,000 October 2022 Pre-Funded Warrants to purchase 3,600,000 common shares at an exercise price of $0.0001 per share, and 5,250,000 Warrants to purchase 5,250,000 common shares at an exercise price of $1.22 per share, in connection with the October 2022 Offering. |
| · | On October 7, 2022, in connection with the October 2022 Offering, we amended the exercise price of 910,000 Amended IPO Common Share Purchase Warrants from $4.77 to $1.17. |
Other than the issuances in connection with the U.S. IPO Offering on October 15, 2021 and the October 2022 Offering on October 7, 2022 to which this registration statement relates, none of the foregoing transactions involved any underwriters, underwriting discounts or commissions or any public offering. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
II-5
Item 8. Exhibits and Financial Statement Schedules
The exhibits listed in the exhibits index, appearing elsewhere in this Registration Statement, have been filed as part of this Registration Statement.
All schedules have been omitted because they are not required, are not applicable or the information is otherwise set forth in the financial statements and related notes thereto.
II-6
II-7
II-8
| 107** | Filing Fee Table |
* Filed herewith.
** Previously filed.
# Indicates management contract or compensatory plan.
†Certain information in this exhibit has been excluded from the version of this document filed as an exhibit because it is both not material and the type of information that the Company treats as private or confidential
Item 9. Undertakings
The undersigned hereby undertakes:
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Filing Fee Tables,” or “Calculation of Registration Fee” table, as applicable, in the effective registration statement;
iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) To file a post-effective amendment to the registration statement to include any financial statements required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be furnished, provided that the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (a)(4) and other information necessary to ensure that all other information in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration statements on Form F-3, a post-effective amendment need not be filed to include financial statements and information required by Section 10(a)(3) of the Act or Rule 3-19 of this chapter if such financial statements and information are contained in periodic reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the Form F-3.
(5) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
i. If the registrant is relying on Rule 430B:
A. Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
II-9
B. Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness of the date of the first contract or sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or
ii. If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(6) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell securities to such purchaser:
i. Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
ii. Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
iii. The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
iv. Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the U.S. Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
II-10
(c) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-11
Pursuant to the requirements of the Securities Act of 1933, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this Post-Effective Amendment No. 2 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Calgary, Province of Alberta, Canada, on August 4, 2023.
| XORTX Therapeutics Inc. | |||
| By: | /s/ Allen Davidoff | ||
| Name: | Allen Davidoff | ||
| Title: | President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this Post-Effective Amendment No. 2 to the Registration Statement has been signed by the following persons in the capacities set forth below on August 4, 2023.
| Signatures | Title | |
| /s/ Allen Davidoff | President and Chief Executive Officer and Director | |
| Allen Davidoff | (Principal Executive Officer) | |
| /s/ James Fairbairn | Interim Chief Financial Officer | |
| James Fairbairn | (Principal Financial Officer and Principal Accounting Officer) | |
| * | Director | |
| William Farley | ||
| * | Director | |
| Anthony Giovinazzo | ||
| * | Director | |
| Ian Klassen | ||
| * | Director | |
| Raymond Pratt | ||
| * | Director | |
| Paul Van Damme |
| *By: | /s/ Allen Davidoff | |||
| Name: | Allen Davidoff | |||
| Title: | Attorney-in-fact | |||
AUTHORIZED REPRESENTATIVE
Pursuant to the requirements of the Securities Act of 1933, as amended, the undersigned certifies that it is the duly authorized United States representative of the registrant and has duly caused this Post-Effective Amendment No. 2 to the Registration Statement on Form F-1 to be signed by the undersigned, thereunto duly authorized, on August 4, 2023.
| PUGLISI & ASSOCIATES | |||
| By: | /s/ Donald J. Puglisi | ||
| Name: | Donald J. Puglisi | ||
| Title: | Managing Director | ||