
Exhibit 99.2

R e d e f i n i n g K i d n e y D i s e a s e January 10th, 2022 Investor Presentation : XR TX : ANU 1

This company presentation may include “forward - looking statements . ” All forward - looking statements are subject to a number of risks, uncertainties and assumptions, and you should not rely upon forward - looking statements as predictions of future events . You can identify forward - looking statements by words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “will,” “plan,” “potential,” “predict,” “project,” “should,” “would” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes . Examples of forward looking statements contained in this presentation include, among others, statements regarding our ability to develop and commercialize XRx - 008 ; our ability to develop other products in our pipeline ; status, timing and results of preclinical studies and clinical trials ; the potential benefits of XRx - 008 ; the timing of seeking regulatory approval of XRx - 008 ; our ability to obtain and maintain regulatory approval ; our estimates regarding our capital requirements ; our plans to develop and market XRx - 008 and the timing of our development programs ; our estimates of the size of the potential markets for XRx - 008 ; sources of cash, including the proceeds from this equity issuance and contributions from other sources ; the rate and degree of market acceptance of XRx - 008 ; our intellectual property position ; our ability to maintain and protect our intellectual property rights ; our results of operations, financial condition, liquidity, prospects, and growth strategies ; our spending of the proceeds from this offering ; the industry in which we operate ; and the trends that may affect the industry or us . This presentation discusses product candidates that are under pre - clinical study and clinical trial and that have not yet been approved for marketing by the Food and Drug Administration . No representation is made as to the safety or efficacy of these product candidates for the therapeutic use for which they are being studied . All forward - looking statements are based upon current estimates and expectations about future events and financial and other trends . There is no guarantee that future results, performance or events reflected in the forward - looking statements will be achieved or occur . Except as required by law, we undertake no obligation to update any forward - looking statements whether as a result of any new information, future events, changed circumstances or otherwise . F o r w a r d L oo ki n g S t a t e m e n ts 2
Developing Novel Therapies to Address the Treatment Needs of P a ti e n t s wi t h A D P K D a n d K i d n e y D i se a s e Ass o c i a t e d w i t h C O V I D - 19 Ou r M i ss i on 3

▪ The global end stage renal disease market was valued at USD 74.5 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 12.7% from 2021 to 2028 (1) . ▪ Developing drug - based therapies for serious progressive kidney diseases with a high unmet medical need, including ADPKD, T2DN and AKI due to COVID - 19. ▪ Three patent families are based upon small molecules and derived from our proprietary pipeline - in - a - product technology with broad therapeutic claims. Multiple studies characterizing the mechanism of action (MoA) in over 700 patients treated, including multiple Ph2 studies allowing to start of Ph3 registration trials; ▪ Senior team was responsible for Oxypurinol development in prior ventures and our key opinion leader - Dr . Richard J . Johnson Jr . , MD, a globally recognized kidney researcher, professor, Nephrologist at the University of Colorado School of Medicine . Management team satisfies new diversity “matrix” . I n v e s t m e n t S u mma r y 4 for Autosomal Dominant Polycystic Kidney Disease (ADPKD > 140 k patients in the US) where there are very limited treatment options and expected to initiate Ph3 pivotal registration clinical trial within 12 months AKI due to Coronavirus infection/ COVID - 19 (WW Pandemic) for type 2 Diabetic Nephropathy (T2DN > 12 m patients in the US) Source: (1) End Stage Renal Disease Market Size, Jan 2021, GVR
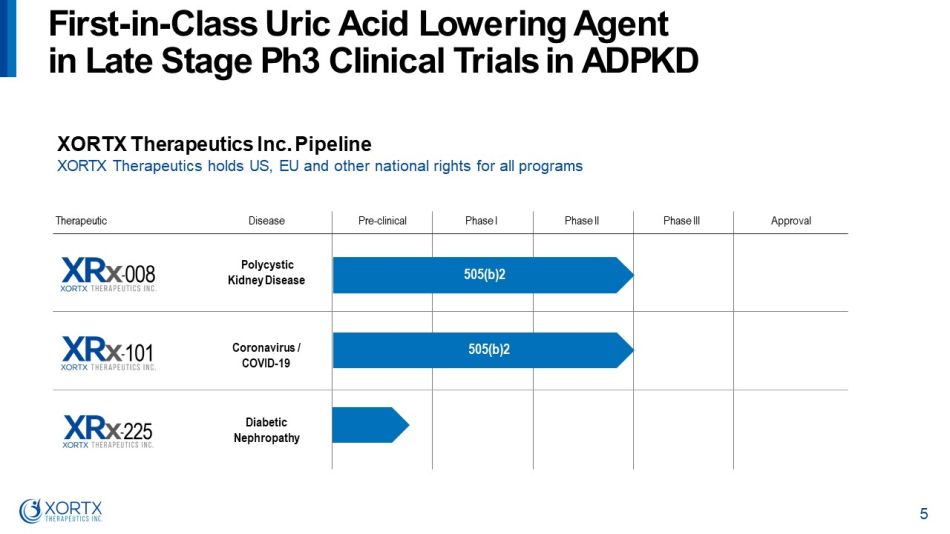
Therapeutic Disease Pre - clinical Phase I Phase II Phase III Approval Polycystic Kidney Disease Coronavirus / COVID - 19 Diabetic N eph r opa t hy XORTX Therapeutics Inc. Pipeline XORTX Therapeutics holds US, EU and other national rights for all programs 505(b)2 505(b)2 F i r s t - in - C l a s s U r i c A c i d L o w e r i n g A gent i n L a t e S t a g e P h 3 Cli n i c a l T r i a l s i n A D P KD 5
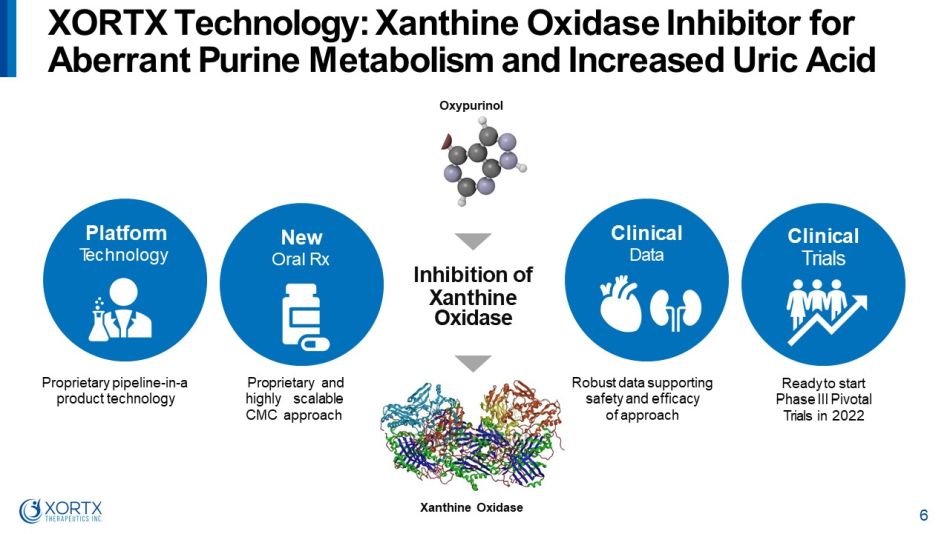
Platform T e c hno l o g y Proprietary pipeline - in - a product technology Proprietary and highly scalable CMC approach Robust data supporting safety and efficacy of approach Ready to start Phase III Pivotal Trials in 2022 XORTX Technology: Xanthine Oxidase Inhibitor for A b e rr a n t P u r i n e M e t a b o li s m a n d I n c r e a s e d U r i c A c i d New Oral Rx Clinical Data Clinical Trials I nh ibit i o n o f X a nt hi ne Oxidase 6 Oxypurinol Xanthine Oxidase
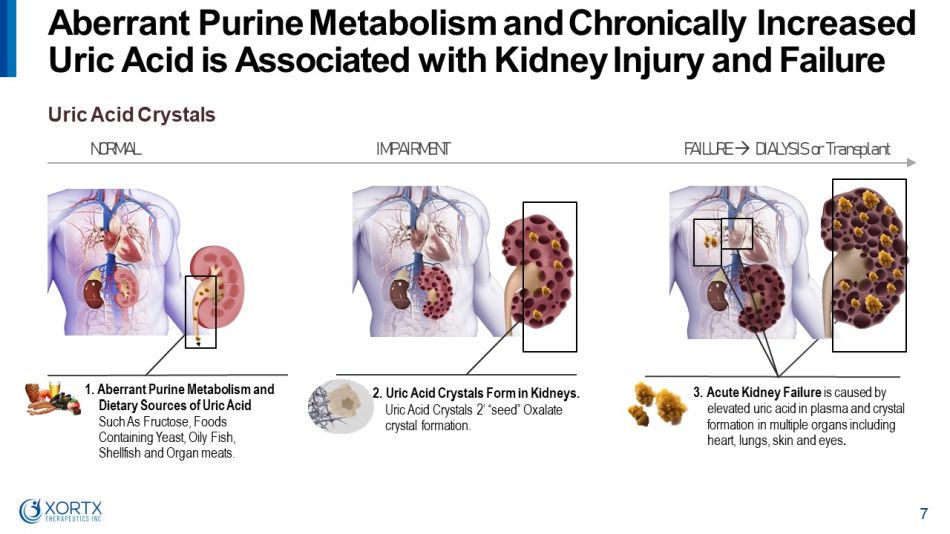
1. Aberrant Purine Metabolism and Dietary Sources of Uric Acid Such As Fructose, Foods Containing Yeast, Oily Fish, Shellfish and Organ meats. 2. Uric Acid Crystals Form in Kidneys. Uric Acid Crystals 2’ “seed” Oxalate crystal formation. 3. Acute Kidney Failure is caused by elevated uric acid in plasma and crystal formation in multiple organs including heart, lungs, skin and eyes . Uric Acid Crystals N O R M A L I M P A I R M E N T F A I L U R E D I AL Y S I S o r T r a n s p l a n t Aberrant Purine Metabolism and Chronically Increased Uric Acid is Associated with Kidney Injury and Failure 7
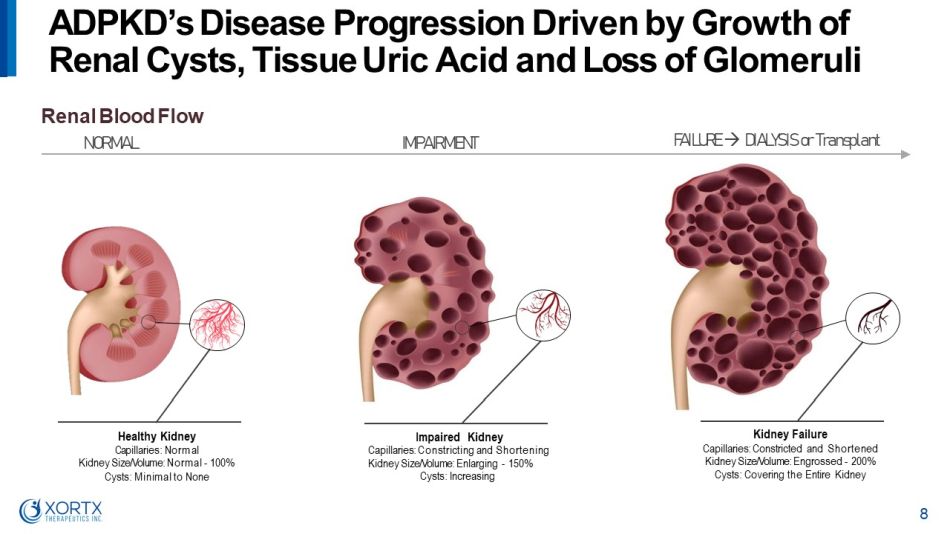
Healthy Kidney C a pill a r ie s : N or m a l Kidney Size/Volume: Normal - 100% Cysts: Minimal to None Impaired Kidney Capillaries: Constricting and Shortening Kidney Size/Volume: Enlarging - 150% Cysts: Increasing Renal Blood Flow NORMAL IMPAIRMENT Kidney Failure Capillaries: Constricted and Shortened Kidney Size/Volume: Engrossed - 200% Cysts: Covering the Entire Kidney ADPKD’s Disease Progression Driven by Growth of Renal Cysts, Tissue Uric Acid and Loss of Glomeruli 8 F A I L U R E D I AL Y S I S o r T r a n s p l a n t
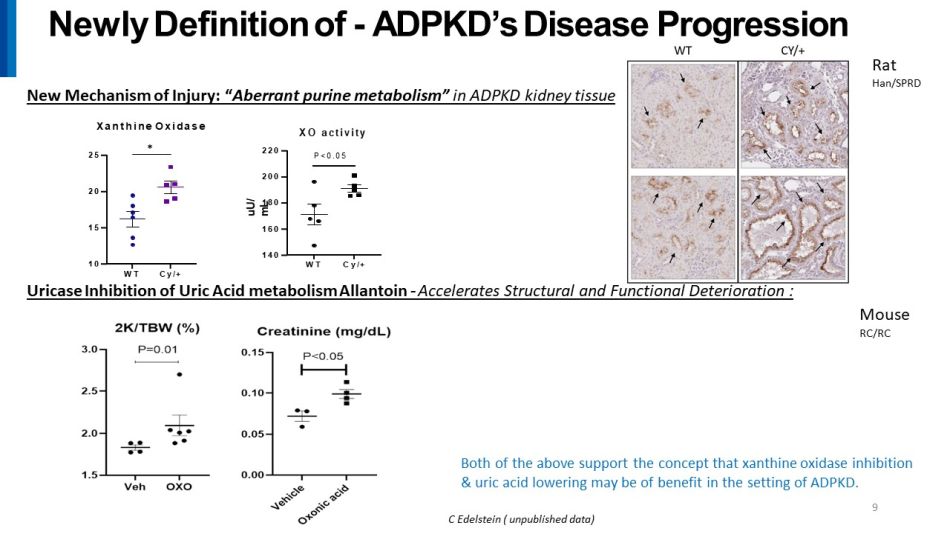
Both of the above support the concept that xanthine oxidase inhibition & uric acid lowering may be of benefit in the setting of ADPKD. 9 C Edelstein ( unpublished data) Newly Definition of - ADPKD’s Disease Progression New Mechanism of Injury: “ Aberrant purine metabolism” in ADPKD kidney tissue W T C y / + 10 15 20 25 Xanthine Oxidase ٓ WT C y / + 1 40 1 60 1 80 2 00 2 20 XO activity uU / m L P <0 . 05 Uricase Inhibition of Uric Acid metabolism Allantoin - Accelerates Structural and Functional Deterioration : Mo use RC/RC Rat Han/SPRD
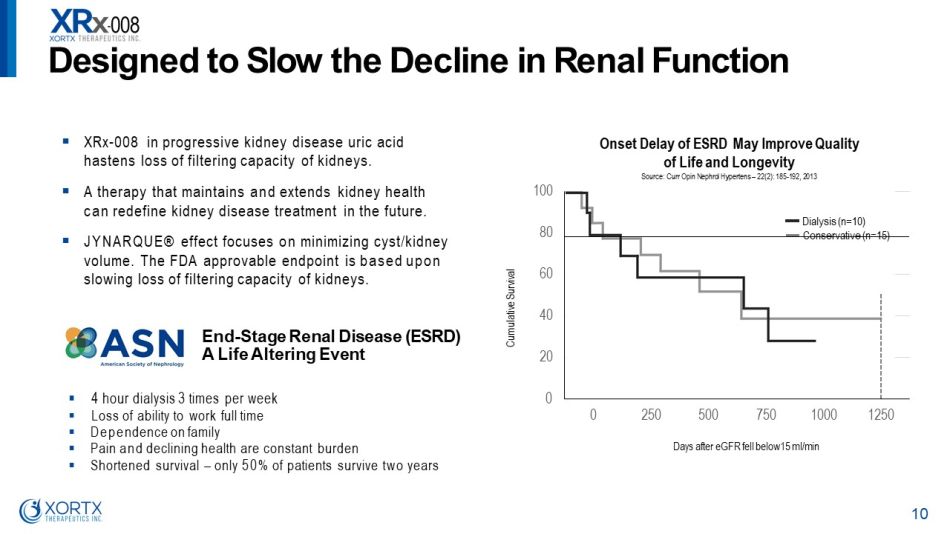
▪ 4 hour dialysis 3 times per week ▪ Loss of ability to work full time ▪ Dependence on family ▪ Pain and declining health are constant burden ▪ Shortened survival – only 50% of patients survive two years ▪ XRx - 008 in progressive kidney disease uric acid hastens loss of filtering capacity of kidneys. ▪ A therapy that maintains and extends kidney health can redefine kidney disease treatment in the future. ▪ JYNARQUE® effect focuses on minimizing cyst/kidney volume. The FDA approvable endpoint is based upon slowing loss of filtering capacity of kidneys. End - Stage Renal Disease (ESRD) A Life Altering Event C umulative S u r vival 50 0 75 0 10 0 0 Days after eGFR fell below15 ml/min Onset Delay of ESRD May Improve Quality of Life and Longevity Source: Curr Opin Nephrol Hypertens – 22(2): 185 - 192, 2013 D es i gne d t o S l o w t h e D e c li n e i n R en a l F unc t i o n 10 0 20 40 60 80 100 0 250 12 5 0 Dialysis (n=10) Conservative (n=15)
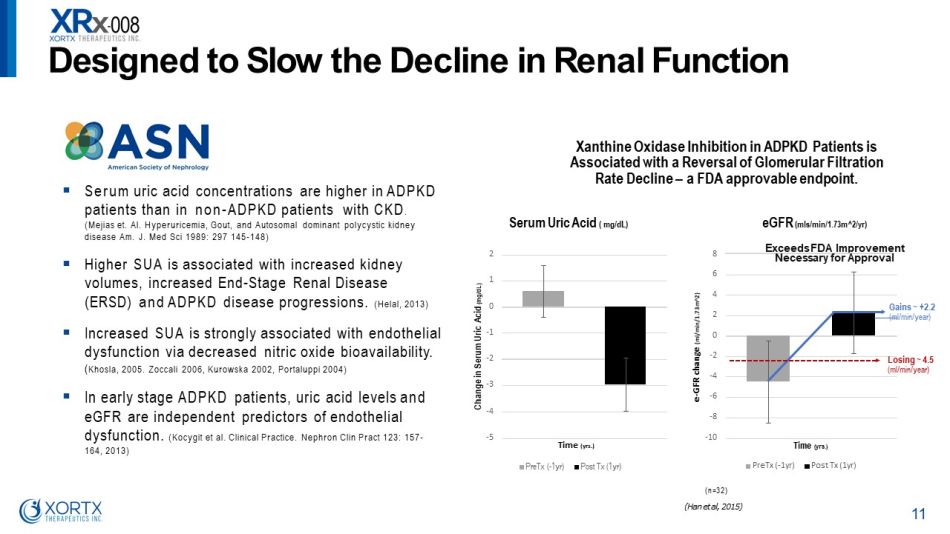
Xanthine Oxidase Inhibition in ADPKD Patients is Associated with a Reversal of Glomerular Filtration Rate Decline – a FDA approvable endpoint. ▪ Serum uric acid concentrations are higher in ADPKD patients than in non - ADPKD patients with CKD . (Mejias et. Al. Hyperuricemia, Gout, and Autosomal dominant polycystic kidney disease Am. J. Med Sci 1989: 297 145 - 148) ▪ Higher SUA is associated with increased kidney volumes, increased End - Stage Renal Disease (ERSD) and ADPKD disease progressions. (Helal, 2013) ▪ Increased SUA is strongly associated with endothelial dysfunction via decreased nitric oxide bioavailability. ( Khosla, 2005. Zoccali 2006, Kurowska 2002, Portaluppi 2004) ▪ In early stage ADPKD patients, uric acid levels and eGFR are independent predictors of endothelial dysfunction. (Kocygit et al. Clinical Practice. Nephron Clin Pract 123: 157 - 164, 2013) (n = 32 ) D es i gne d t o S l o w t h e D e c li n e i n R en a l F unc t i o n 11 ( H a n e t a l , 2015 ) - 10 - 8 - 6 - 4 - 2 0 2 4 6 8 e - GFR change (ml/min/1.73m^2) T i m e ( y r s . ) eGFR (mls/min/1.73m^2/yr) PreTx ( - 1yr) Post Tx (1yr) - 5 - 4 - 3 - 2 - 1 0 1 2 C h an g e in S e r u m U r ic A cid ( mg/dL) Serum Uric Acid ( mg/dL) Time (yrs.) PreTx ( - 1yr) Post Tx (1yr) Gains ~ +2.2 (m l/min/year) E xce ed s F DA I m p r o v em e n t N e c e ss a r y f o r A pp rov a l Losing ~ 4.5 (ml/min/year)
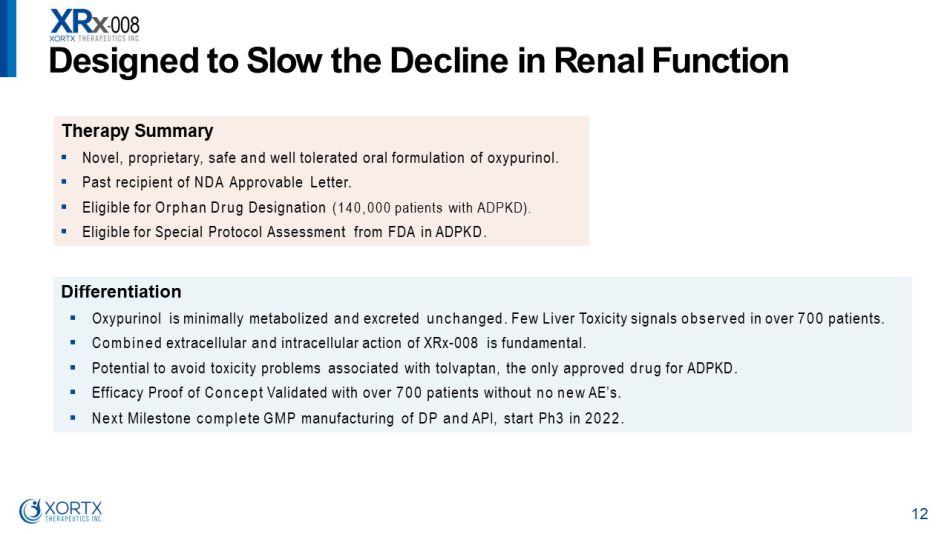
T h e r a p y S u mm ar y ▪ Novel, proprietary, safe and well tolerated oral formulation of oxypurinol. ▪ Past recipient of NDA Approvable Letter. ▪ Eligible for Orphan Drug Designation (140,000 patients with ADPKD). ▪ Eligible for Special Protocol Assessment from FDA in ADPKD. Differentiation ▪ Oxypurinol is minimally metabolized and excreted unchanged. Few Liver Toxicity signals observed in over 700 patients. ▪ Combined extracellular and intracellular action of XRx - 008 is fundamental. ▪ Potential to avoid toxicity problems associated with tolvaptan, the only approved drug for ADPKD. ▪ Efficacy Proof of Concept Validated with over 700 patients without no new AE’s. ▪ Next Milestone complete GMP manufacturing of DP and API, start Ph3 in 2022. D es i gne d t o S l o w t h e D e c li n e i n R en a l F unc t i o n 12
▪ 140,000 patients diagnosed with ADPKD in the US (1) ▪ ADPKD is the largest kidney disease market with a genetic origin ▪ A majority of ADPKD patients require dialysis or kidney transplantation ▪ Otsuka's JYNARQUE® (tolvaptan) was approved in 2018 for the treatment of AKPKD with black box warning ▪ Annual Treatment Cost of JYNARQUE® (tolvaptan) ~$156,000. Otsuka reported 2020 sales of tolvaptan of $620 m, More than 5,000 ADPKD patients have been treated with tolvaptan (2) ▪ 85% of ADPKD patients can’t take or tolerate JYNARQUE® (tolvaptan) (3) A D P K D – On l y On e T he r a p y i s A pp r o v e d W h i l e Suboptimal Treatment Options Remain 13 Sources: (1) Vicente E. Torres March 2021 AJKD , (2) Otsuka , (3) Wim Van Biesen, Jan 2019, NDT
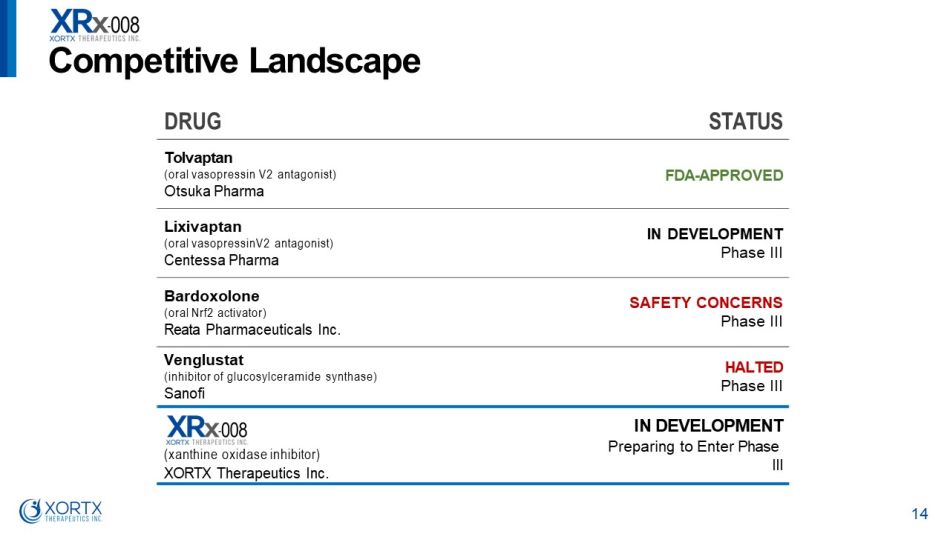
DRUG STATUS Tolvaptan (oral vasopressin V2 antagonist) Otsuka Pharma FDA - APPROVED Lixivaptan (oral vasopressinV2 antagonist) Centessa Pharma I N D E V E L O P M ENT P h a s e II I Bardoxolone (oral Nrf2 activator) Reata Pharmaceuticals Inc. S A FE T Y CONCE R NS P h a s e II I Venglustat (inhibitor of glucosylceramide synthase) Sanofi HALTED P h a s e II I (xanthine oxidase inhibitor) XORTX Therapeutics Inc. IN DEVELOPMENT Preparing to Enter Phase III Com p e t i t i v e L a nds c a p e 14
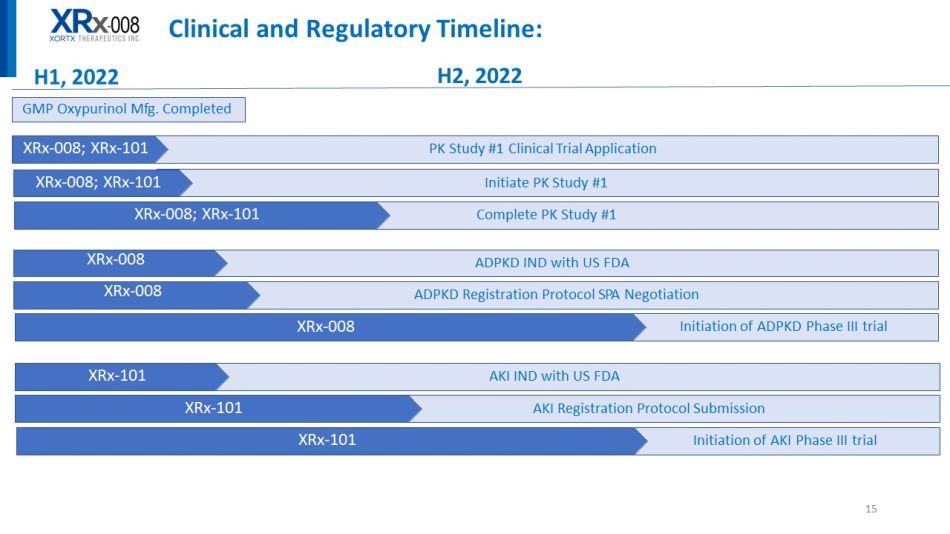
Initiation of ADPKD Phase III trial 15 Clinical and Regulatory Timeline: H1, 2022 H2, 2022 GMP Oxypurinol Mfg. Completed PK Study #1 Clinical Trial Application XRx - 008; XRx - 101 Initiate PK Study #1 XRx - 008; XRx - 101 Complete PK Study #1 XRx - 008; XRx - 101 ADPKD IND with US FDA ADPKD Registration Protocol SPA Negotiation XRx - 008 X R x - 008 X R x - 008 Initiation of AKI Phase III trial AKI IND with US FDA X R x - 101 AKI Registration Protocol Submission X R x - 101 X R x - 101
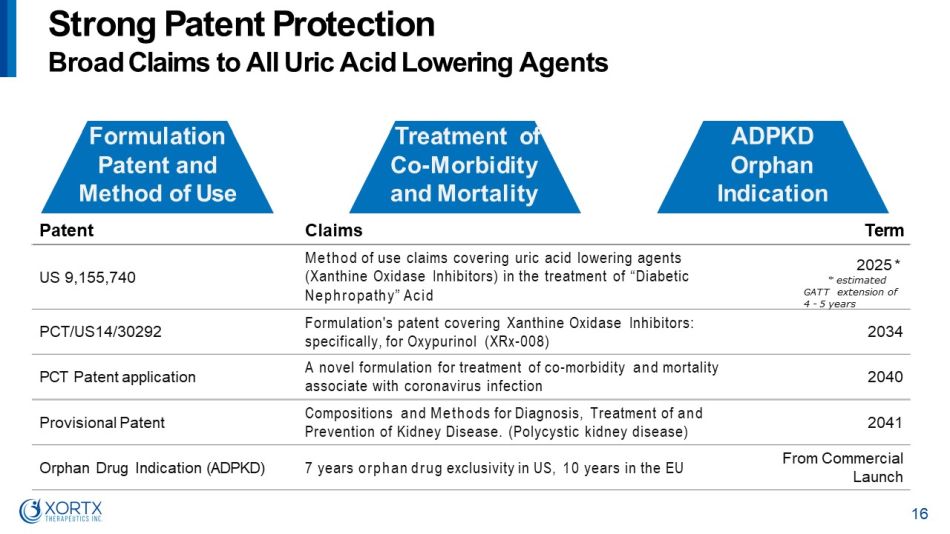
Patent Claims Term US 9,155,740 Method of use claims covering uric acid lowering agents (Xanthine Oxidase Inhibitors) in the treatment of “Diabetic Nephropathy” Acid 2025 * * es t i ma t ed G A TT e xt e n s i on of 4 - 5 years PCT/US14/30292 Formulation's patent covering Xanthine Oxidase Inhibitors: specifically, for Oxypurinol (XRx - 008) 2034 PCT Patent application A novel formulation for treatment of co - morbidity and mortality associate with coronavirus infection 2040 Provisional Patent Compositions and Methods for Diagnosis, Treatment of and Prevention of Kidney Disease. (Polycystic kidney disease) 2041 Orphan Drug Indication (ADPKD) 7 years orphan drug exclusivity in US, 10 years in the EU From Commercial Launch Formulation Patent and Method of Use S t r o n g P a t e n t P r o t e c t i o n Broad Claims to All Uric Acid Lowering Agents Treatment of Co - M o r b i d i t y and Mortality ADPKD Orphan I n d ic a ti o n 16
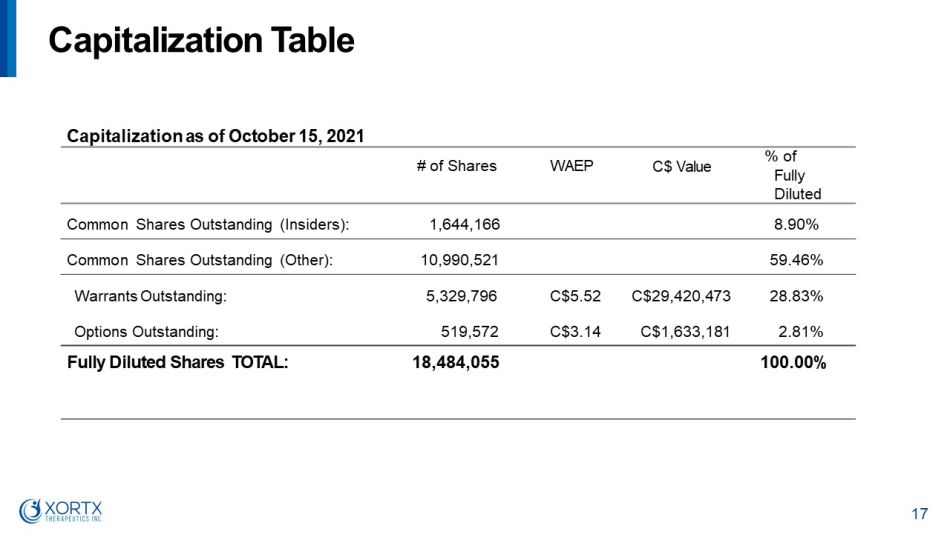
C a p i t a l i z a t i o n T a b le 17 C a p i t a l i z a t i o n a s of O c t obe r 1 5 , 2 0 2 1 # of Shares WAEP C$ Value % o f F u l l y Diluted Common Shares Outstanding (Insiders): 1,644,166 8.90% Common Shares Outstanding (Other): 10,990,521 59.46% W a rr a n t s Ou t s t a nd i ng : 5,329,796 C$5.52 C$29,420,473 28.83% Options Outstanding: 519,572 C$3.14 C$1,633,181 2.81% F u l l y D i l u t e d S ha r e s T O T AL: 18,484,055 100.00%

Dr. Allen W. Davidoff, Ph.D. Chief Executive Officer Dr . Allen Davidoff has over 19 years drug development experience) is the founder and CEO of XORTX . Allen has a broad range of clinical and regulatory experience and senior management experience in pharmaceutical R&D including four investigational new drug (“IND”) applications or supplemental IND’s, two phase I studies (four of which were multi - country), eight phase II studies, and one NDA . Prior to co - founding XORTX, Allen was the Chief Scientific Officer, VP Product Development and co - founder of Stem Cell Therapeutics Corp . (seven years) Trillium TRIL : NASDAQ acquired by Pfizer and Senior Scientist and Head of Pharmacology at Cardiome Pharma Corp . Amar Keshri, CPA Chief Financial Officer Amar Keshri has over 15 years accounting experience in a number of sectors including “life science” and “oil and gas” industries through public practice audit and finance and accounting consulting roles including with Suncor, PricewaterhouseCoopers LLP and Ernst & Young . Mr . Keshri is a Member of the Institute of Chartered Accountants of Alberta . Dr. Stephen Haworth Chief Medical Officer Dr . Haworth has over 25 + years of successful global drug development and senior leadership in both “start - up“ and Fortune 500 pharmaceutical firms in both the US and Europe . Stephen has a broad clinical and regulatory experience that ranges from infectious disease through nephrology, cardiovascular disease and most recently on programs for treatment and prevention of SARS - CoV infection . He has held key roles in numerous FDA and EMA submissions and has been involved in several licensing and M&A transactions . Dr . Haworth holds a medical degree from University College Hospital Medical School, University of London, having graduated with Honors . M a n a g e m e n t T e a m 18

Paul Van Damme B.COMM., CPA, MBA - Chairman Held senior positions with a number of Canadian and US public companies . His experience focused on the biotech industry in Toronto when he joined GlycoDesign, a private biotech company . While at Allelix Pharmaceuticals Inc . , he participated in the sale of that company to NPS P h a r m ac e u t i c a l s , I n c . D r . All en W . D a v i d o f f P h . D . – P r esi d en t a n d CE O Over 19 years drug development experience) is the founder and CEO of XORTX . Allen has a broad range of clinical and regulatory experience and senior management experience in pharmaceutical R&D including four investigational new drug (“IND”) applications or supplemental IND’s, two phase I studies (four of which were multi - country), eight phase II studies, and one NDA . Prior to co - founding XORTX, Allen was the Chief Scientific Officer, VP Product Development and co - founder of Stem Cell Therapeutics Corp . (seven years) Trillium TRIL : NASDAQ acquired by Pfizer and Senior Scientist and Head of Pharmacology at Cardiome Pharma Corp . Dr . Raymond Pratt – Raymond Pratt is an accomplished Physician Executive in clinical medicine, Nephrology, drug development, and the pharmaceutical industry . He has extensive experience troubleshooting issues concerning regulatory approval of drugs and devices and providing development strategies for global pharmaceutical companies . William Farley Over 35 years experience in the Business Development, Sales and leading efforts in drug discovery, development and partnering . Prior to joining the board of directors of XORTX, Mr . Farley held a senior leadership position at Sorrento Therapeutics . Bill, began his career at Johnson and Johnson and has also held senior management positions at Pfizer and HitGen Ltd . and Vice President, WuXi Apptec, Inc . creating, building and leading global business development teams, and Vice President, Business Development at ChemDiv where he led numerous efforts to create new therapeutic companies in CNS, Oncology and Anti infectives . Ian Klassen Brings almost 30 years of business management, public relations and government affairs experience . He previously served as Chief of Staff to the Canadian Speaker of the House of Commons . Ian is the recipient of the Commemorative Medal for the 125 th Anniversary of the Confederation of Canada in recognition of his significant contribution to his community and country . Jac q u el i n e L e S a u x A seasoned Canadian health care legal executive who has held senior positions at large and small public and private life science companies . Jacqueline’s legal experience is focused on securities, pharmaceutical regulatory and intellectual property law . As a Vice President, Legal in both public and private companies Ms . Le Saux has led multiple financings, mergers and acquisitions and product licensing transactions . 19 Bo a r d o f D i r e ct o r s

Dr. Petter Bjornstad, M.D . University of Colorado Denver School of Medicine Barbara Davis Center. Dr. Richard Johnson, M.D Professor of Medicine and the Chief of the Renal Division and Hypertension at the University of CO. Dr. Anjay Rastogi, M.D., Ph.D . Professor and Clinical Chief of Nephrology at the David Geffen School of Medicine at UCLA, Los Angeles, CA. Dr. Federico Maese, M.D. Cardiology Specialist in Red Oak, TX. Dr. Henk ter Keurs, M.D. Professor of Cardiac Sciences, Medicine at the University of Calgary. Dr. Charles Edelstein, M.D., Ph.D., Professor of Medicine and Nephrologist at the University of Colorado . Dr . Edelstein is board certified in Nephrology and has a doctoral degree (PhD) in Internal Medicine . He did his Internal Medicine residency and Nephrology fellowship at University of Stellenbosch and University Cape Town Medical School, respectively . His academic research focuses on both therapeutic studies in animal models of polycystic kidney disease (PKD) as well as acute kidney injury (AKI) and biomarkers of AKI . Dr . Edelstein is a world leader in PKD research and PKD care and has received an award for the WSCI Outstanding Investigator Award and is a former president of the Western Section of the American Federation of Clinical Research and International Society of nephrology member and American Society of Nephrology Advisory Committee Member . 20 Cli n i c a l A d vis o r y B o a r d

▪ The global end stage renal disease market was valued at USD 74.5 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 12.7% from 2021 to 2028. ▪ XORTX is developing a therapeutic solution for ADPKD, with a unique mechanism of action, focused on slowing the progression of kidney disease. for ADPKD may improve kidney function. ▪ The successful completion of our Ph3 trial in ADPKD will provide optimal information for registration with the FDA in pursuit of marketing approval in the US . XORTX’s technologies are supported by management’s disciplined strategy to generate IP, clinical data, reimbursement, manufacturing and commercial infrastructure I n v e s t m e n t S u mma r y U r i c A c i d L o w e r i n g A gen t s U s e i n K i dn e y D i se a se 21

Cont ac t U s X O R T X T h e r a p e ut i c s I n c . 4000, 421 7th Avenue SW Calgary, Alberta T2P 4K9 Ph: +1 (403) 455 - 7727 i n f o @ x o r t x . c om ww w . x o r t x . c om X O R T X T her a p e u t ic s I nc. R ed e f i n i n g K i dne y D i s e a s e 22 : XR TX : ANU 1