Technology development is an integral part of our corporate strategy. XORTX Therapeutics is working with a number of development and technological partners with proven competence in manufacturing, non-clinical and clinical program development. Importantly, contract research organizations with experience in clinical and regulatory development as well as an orphan drug focus. Our common goal is to bring novel therapies to market to address the needs of both patients and physicians.
XORTX is developing two separate products to treat progressive kidney disease in patients with autosomal dominant polycystic kidney disease (ADPKD) or diabetic nephropathy (DN) and one product XRx-101 to reduce the acute kidney injury associated with coronavirus/ COVID-19 infection. The Company’s ADPKD program -XRx-008 – is novel formulation of xanthine oxidase inhibitor designed to slow or reverse disease progression in this rare disease. Our core compound is Oxypurinol, a drug that has been extensively studied and characterized as safe and effective for inhibiting xanthine oxidase and decreasing uric acid in a patient’s blood. Recent clinical trials results have shown that uric acid concentration in the blood is an independent risk factor for patients with ADPKD. A therapy that decreases the influence of aberrant purine metabolism and uric acid has the potential to slow the rate deterioration of kidney function and potentially redefine therapeutic options for this disease. XORTX is also evaluating several drug candidates for use in treating diabetic nephropathy under the XRx-225 program and in advancing this program to clinical trials.
Pilot clinical studies published in recent years, suggests that blood concentrations of uric acid may be a causative in hypertension, inflammation, metabolic syndrome, diabetes and health consequences of diabetes such as diabetic nephropathy/kidney injury. The Company’s patent portfolio, and similarly this therapeutic approach, continues to be validated in a number of independent studies that show that by decreasing serum uric acid (SUA) can reduce markers of inflammation, progression of kidney injury including proteinuria, glomerular filtration rate, hypertension, insulin resistance, and chronic kidney disease, in a clinically meaningful way. XORTX is dedicated to growing its patent portfolio to strengthen and broaden the Company’s position in this field.
Aberrant Purine Metabolism and Chronically Increased Uric Acid is Associated with Kidney Disease and Failure

Autosomal Dominant Polycystic Kidney Disease (ADPKD)
ADPKD is an orphan indication disease affecting an estimated 1:500-1:2000 individuals and a leading cause of end stage kidney renal disease. It is a disease representing up to 5% of all patients who have kidney failure before the age of 60 years and is amongst the fastest progressing forms or kidney disease.
Evidence from a variety of recent studies has accumulated, supporting the concept that aberrant purine metabolism and increased serum uric acid (SUA), when increased above the upper limit of normal is a “causative” mediator of hypertension, with additional evidence suggesting a role of hyperuricemia (elevated serum uric acid) in the development of endothelial dysfunction, insulin resistance, diabetes and diabetic nephropathy.
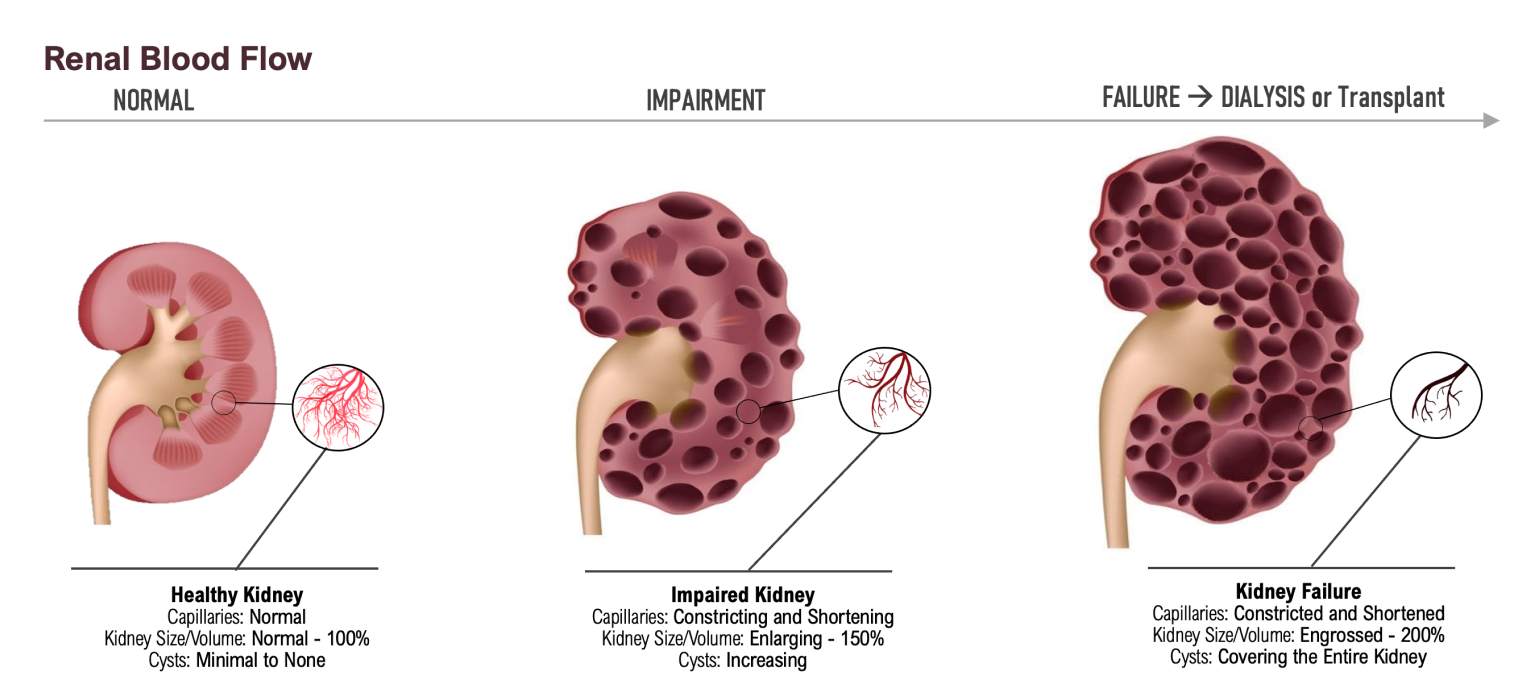
ADPKD's Disease Progression Driven by Rupture of Renal Cyst, elevated Uric Acid and Loss of Capillarity
ADPKD - Only One Therapy is Approved While Suboptimal Treatment Options Remain

- 140,000 patients diagnosed with ADPKD in the US(1)
- ADPKD is the largest kidney disease market with a genetic origin
- A majority of ADPKD patients require dialysis or kidney transplantation
- Otsuka's JYNARQUE® (tolvaptan) was approved in 2018 for the treatment of AKPKD with black box warning
- Annual Treatment Cost of JYNARQUE® (tolvaptan) ~$156,000. Otsuka reported 2020 sales of tolvaptan of $620 m. More than 5,000 ADPKD patients have been treated with tolvaptan(2)
- 85% of ADPKD patients can't take or tolerate JYNARQUE® (tolvaptan)(3)
Diabetic Nephropathy- A Health Consequence of Diabetes
Diabetes is prevalent worldwide and represents and EPIDEMIC in the US:
- According to the CDC, diabetes affected 11.3% (~25.6 Million) of the U.S. population age 20 or older in 2010, with about 1.9 million people being newly diagnosed each year (2).
- In 2005–2008, based on fasting glucose or hemoglobin A1C levels, 35% of U.S. adults aged 20 years or older had pre-diabetes (metabolic syndrome) (2).
- Diabetic nephropathy is the most common cause of chronic kidney failure and end-stage kidney disease in the United States and worldwide.
- In the U.S., diabetic nephropathy accounts for about 45% of new cases of ESRD(3).
- About 30% of patients with type I or type II diabetes develop evidence of nephropathy (3).
The company is actively reviewing current studies characterizing the outbreak of SARS-CoV-2 (COVID-19). These published reports clearly illustrate that acute kidney injury (AKI) and acute, multiple, organ injury are key factors in the most serious cases of COVID-19 hospitalization and death.
XORTX’s XRx-101 program (a novel formulation of uric acid lowering agent) could be an important therapy with the ability to decrease acute kidney, as well as health consequences associated with Coronavirus infection.
XORTX reported topline results in Nov 2020, that a majority of individuals hospitalized with coronavirus/ COVID-19 showed evidence of co-incidence of uric acid concentrations greater than normal and acute kidney injury. Severity of Acute kidney injury during coronavirus/COVID-19 infection has been associated with worse outcomes and increased mortality.
XRx-101 a Therapy Designed to Treat Coronavirus / COVID-19
XORTX has filed new intellectual property rights for XRx-101 as a treatment aimed at decreasing acute kidney disease and organ injury associated with viral infection.
SARS-CoV-2 (COVID-19) is a serious viral infection due to a coronavirus. Coronavirus infections such as SARS and MERS and specifically COVID-19 can be frequently accompanied by pneumonia, acute kidney injury, proteinuria and hematuria1,2. Acute kidney injury ("AKI") has been identified as an independent risk factor for patients' in-hospital mortality due to COVID-19 as well as other Coronavirus infections2. Early reports suggested a lower incidence (between 3% to 9%) of AKI in those with COVID-19 infection2,6,7. Recent reports, however, have shown a higher frequency of renal abnormalities. A study of 59 patients with COVID-19 found that 34% of patients developed massive albuminuria on the first day of admission and 63% developed proteinuria during their stay in hospital8. Equally concerning, individuals with "Long COVID-19" appear to have increased susceptibility to kidney damage and end-stage kidney disease.10
A NOVEL SOLUTION:
XORTX’s business model is built upon data driven science, and recent clinical science successes, including:
- Animal and epidemiological studies that have demonstrated an association between increased serum uric acid and hypertension (4,5).
- Two completed Phase 2 Trials have demonstrated uric acid lowering agents are effective at reducing blood pressure in new onset adolescent hypertension (6,7).
- Animal and epidemiological studies have demonstrated an association between high serum uric acid insulin resistance (8, 9). A finding that suggest that Xanthine Oxidase Inhibition (XOI) may be an important new option to treat pre-diabetes and diabetes.
- Indeed, evidence continues to emerge suggest that high uric acid has an effect on development and progression of diabetic nephropathy (10).
- In addition, three observational studies in patients with diabetes have generated a substantial body of evidence that serum uric acid levels are strong determinants of the development of diabetic nephropathy and the loss of kidney function among individuals with diabetes, both Type 1 and Type 2 (11,12,13).
- Other randomized controlled trials in patients with CKD or diabetic nephropathy demonstrated xanthine oxidase inhibition can decrease uric acid concentration and significantly improved kidney function (14, 15).
Historically, extensive clinical use of XOIs provides a well characterized basis for developing new therapeutic approaches for underserved medical need. Our increasing understanding of mechanism of injury that may occur in the presence of aberrant purine metabolism and increased serum uric acid, specific to the treatment of kidney disease, and represents an opportunity for developing new medical therapies in areas where few therapeutic options exist.
For more information please contact XORTX Therapeutics Inc.